The pandemic has given us all a little crash course in immunology, but even the experts still have plenty of questions about SARS-CoV-2 protection.
There was a time before covid, which seems a distant memory now, when the concepts of “immune” and “immunised” seemed relatively straightforward to anyone outside the field of immunology.
Either by virtue of childhood infection or vaccination, a person believed that they were now protected from further assault by that particular pathogen. Even the Oxford dictionary defines “immune” as “resistant to a particular infection”.
Along comes SARS-CoV-2 and a global pandemic that has ripped the scales from our eyes and thrust us, whether we like it or not, into the highly complex world of antibodies, antigens, immunoglobulins, B cells and T cells. It has also revealed just how naïve our expectation of immunity was.
The arrival of covid vaccines raised hopes that this would spell the end of the pandemic and bury this pesky virus. But then came the first breakthrough infections, then variants, then third doses, then Omicron.
Now, the pharmaceutical companies behind these blockbuster vaccines are moving to get approval for a second booster dose, amid emerging evidence that this could offer greater protection against severe disease and death, at least for older, at-risk individuals.
Part of the problem is many misunderstand the purpose of vaccines, says Professor Daniela Weiskopf, immunology researcher at the La Jolla Institute for Immunology in California.
“People were very disappointed when they got their two shots and then still got infected,” Professor Weiskopf says. “But you get a vaccine not to protect you from infection, but to keep you out of the hospital.”
On that front, a primary course of vaccines – two doses of Pfizer or AstraZeneca, in the case of most Australians – was remarkably successful at reducing the risk of severe illness, hospitalisation and death, and also pretty effective at preventing symptomatic infection.
So why the need for boosters? Because immunity – at least that which is measurable by antibody levels – always wanes over time.
“It’s very normal for the humoral immune response, which is the antibodies, to contract back to homeostasis,” says Professor Cassandra Berry, professor of immunology at Murdoch University in West Australia.
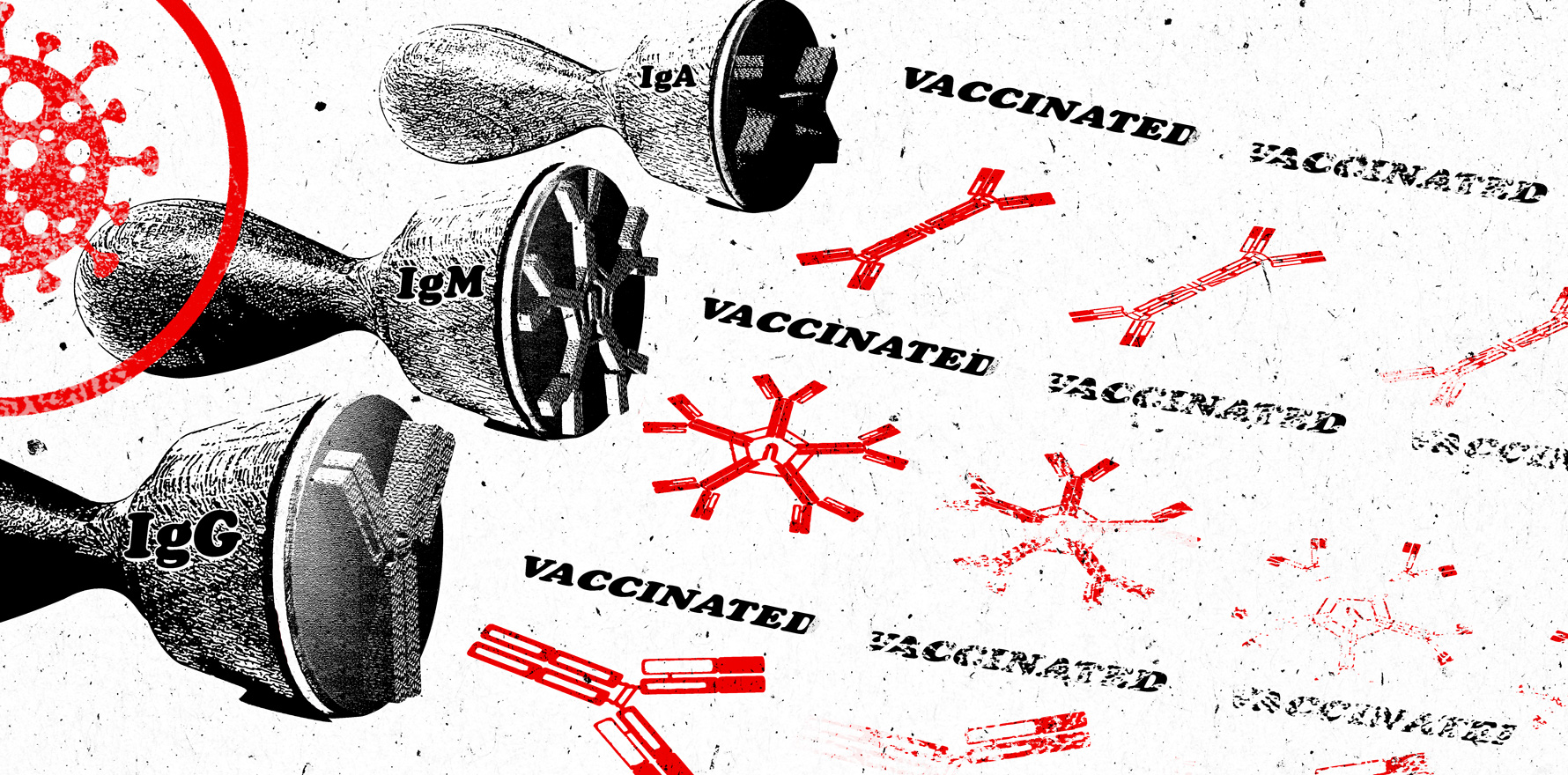
These antibodies – in particular the immunoglobulins IgA, IgM and IgG – wax and wane at different times and rates after infection. IgM antibodies are first to appear – the “shock troops” of the humoral immune response, providing immediate protection – but these become almost undetectable by two to three months after infection. IgA antibodies also appear quickly in the upper respiratory tract and mucosal surfaces, and then vanish within around three months. Finally there are the IgG antibodies, which appear a bit later but which hang around for six to eight months.
When people talking about “waning immunity” they’re talking about the inevitable decline of these antibodies. But that’s normal, Professor Berry says, “because we just don’t have enough room in our bodies to have all these activated B cells churning out antibodies – our lymph nodes would explode.” That’s one reason why the first booster doses were rolled out, and why we’re now potentially looking at second booster doses.
The other reason for boosters is Omicron, Professor Weiskopf says. “Before Omicron, we saw that the immune response was pretty stable; it did certainly not fall off a cliff, not after infection and even less so after vaccination,” she says. “Then Omicron was able to jump over the wall of immunity for antibodies.” Booster doses raise that “wall” of antibodies high enough to keep Omicron out, or at least make it a lot harder for it to infect and cause serious disease.
Antibodies aren’t the only arm of the immune response; they’re just the easiest to measure. The other arm is the cellular immunity – the B cells that generate those antibodies, and the T cells that recognise and destroy cells infected with the virus as well as stimulating the production of antibodies by B cells.
Unfortunately, B cells and T cells are harder to study, because it requires taking a larger amount of blood than for antibody tests, and the cells are harder to store in vitro. This means many of the population-based serosurveys being done around the world aren’t able to examine what happens to immunity after those immunoglobulin antibodies drop off.
There is now a lot of research focusing on what happens to T cells after covid infection or vaccination. The reason for that interest is that T cells don’t prevent infection – that’s the job of the antibodies, the IgA, IgM and IgG – but they are key to dealing with it once it’s happened. Even early in the pandemic, there was evidence that higher levels of T cells meant more of those neutralising antibodies, and lower levels of some types of T cells were associated with worse outcomes.
Another benefit of T cell-mediated immunity to SARS-CoV-2 is that it seems to not be compromised by the different variants. Because T cells seek out and destroy cells infected with the virus by looking for telltale signatures of infection on the cell surface (known as epitopes), they are able to recognise a range of those signatures – around 15 to 20 of them.
So, for an infected cell to completely evade the T cell response, the virus would need to have mutations in exactly those same 15 to 20 signatures to make it undetectable.
“And that has so far not been the case,” Professor Weiskopf says.
But to complicate matters further, T cell immunity to covid isn’t all good, says Dr Anthony Leonardi, a T cell immunologist at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
The first issue is that SARS-CoV-2 seems to cause T cells to infiltrate the brain after infection, “and that does not happen with influenza, it’s not a typical thing,” Dr Leonardi says.
There’s also concern about the role that T cells might be playing in long covid – particularly with neurological symptoms – with one study suggesting that T cells could affect learning, memory and the experience of pain in long covid.
Dr Leonardi is also worried that with repeated covid infection, T cell populations may get depleted and exhausted. “The T cell immunity, even though you have long-lived B cells and T cells, it doesn’t necessarily suffice for all people in prevention of severe illness, especially if you’re aged,” Dr Leonardi says. “What you need are antibodies, and the problem is the antibodies are the ones that fade first.”
So what does the future hold for those antibodies, particularly as SARS-CoV-2 continues to mutate and generate new variants? Professor Berry says that the inevitable waning of antibody levels after vaccination means those who are most vulnerable to severe disease – the elderly, chronically ill and immunocompromised – will likely need a fourth dose to restore their antibody protection.
She also sees covid vaccination as evolving to be something like influenza vaccination, and perhaps even happening in conjunction with it. “We’ll probably have to have annual vaccines, maybe in combination with the flu vaccine,” she says.
Professor Weiskopf also imagines a future in which covid vaccines are offered annually, just like influenza vaccines, either adapted to a new variant or to boost antibody levels against an existing variant. But she says there are still many questions to be answered about what the best covid protection looks like, and how that immunity can be measured.
“There’s other viral diseases or infections where we still don’t know after 20 years what the correlate of protection is,” she says. “We have a very good understanding of six to nine months after infection or vaccination [for covid], but we don’t know what happens in two years.”