UK researchers believe they’ve identified the best risk prediction model, but other experts aren’t sold just yet.
A recent study gives the nod to a model that prioritises sensitivity over specificity when forecasting ovarian cancer.
Many cases of ovarian cancer are diagnosed at an advanced stage, despite there being several different models that can be used to predict a person’s risk.
The fact that this cancer is associated with non-specific symptoms (such as abdominal bloating, unexplained weight loss and changes in the frequency of bowel movements) may contribute to the late diagnosis. It is also not clear what risk prediction model is most accurate.
Now, UK researchers have compared the various risk prediction models in an attempt to identify the best diagnostic test for ovarian cancer.
The findings of the prospective diagnostic accuracy study, published in The Lancet Oncology, suggest the International Ovarian Tumour Analysis consortium’s Assessment of Different Neoplasias in the Adnexa (IOTA ADNEX) should be considered the new gold standard diagnostic test for ovarian cancer in postmenopausal patients.
Researchers recruited 1242 postmenopausal patients who presented to one of 12 hospitals with non-specific symptoms and elevated CA125 levels or abnormal ultrasound findings. Women with a prior history of ovarian cancer were excluded.
Patients completed questionnaires about their symptoms and sociodemographic characteristics (age, height, weight, smoking status, etc.), provided a blood sample, and underwent both a transabdominal and transvaginal ultrasound.
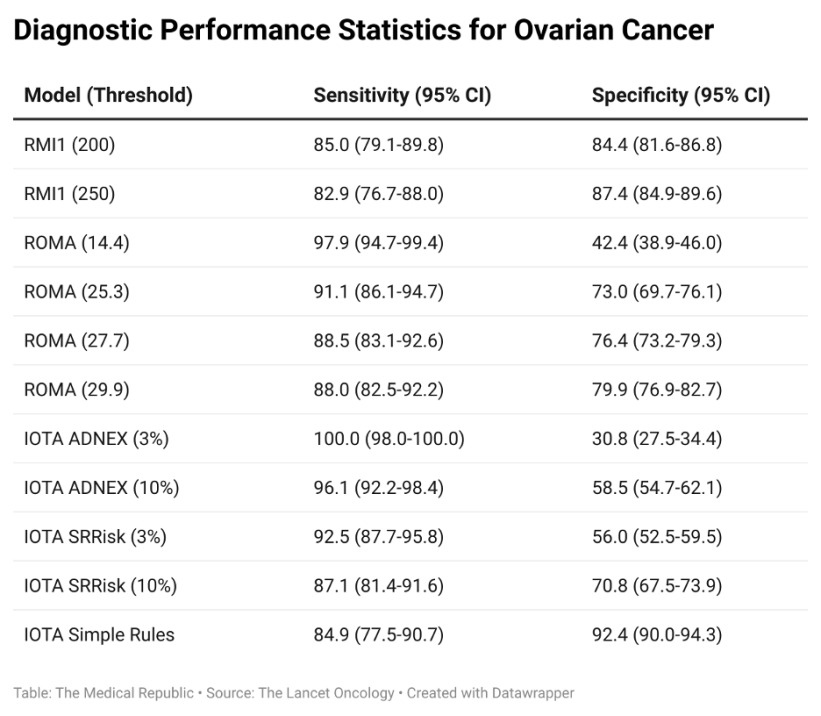
The results of the questionnaires and tests were used to determine ovarian cancer risk according to each of the following models: the Risk of Malignancy Index 1, three separate IOTA models (the ADNEX, the Simple Rules and Simple Rules Risk models), the Risk of Malignancy Algorithm (ROMA) and the CA125 concentration. The RMI1 was used as the reference that the other models were compared to.
The median age of recruited patients was 65.3 years, and 215 (17%) were diagnosed with ovarian cancer within the first 12 months of entering the study. The IOTA ADNEX model was found to have the highest sensitivity when a 3% threshold was used (100%, compared to the 82.9% sensitivity of the RMI1 at a threshold of 250) but had a much lower specificity (30.8% versus 87.4%). Changing the IOTA ADNEX threshold to 10% resulted in a slight drop in sensitivity (96.1%) but increased specificity (58.5%), which led the researchers to recommend the IOTA ADNEX with the increased threshold as the new standard of care.
While the researchers stated that the focus on sensitivity over specificity was supported by participant and patient advocates, along with policy experts, they acknowledged this approach could result in a greater amount of false positive diagnoses, resulting in anxiety for patients and extra work for healthcare systems.
These concerns were echoed by the authors of a commentary that accompanied the manuscript, who felt the researchers were jumping the gun in recommending the IOTA ADNEX as the new standard of care.
“The study’s unique approach to testing all risk prediction models in each individual patient, combined with a meticulous methodology, has numerous strengths. However, several questions still need to be answered,” the commentary authors wrote.
“The authors emphasised that early-stage diagnosis dramatically improves survival. Therefore, providing more details on each model’s ability to predict stage I ovarian cancer or borderline tumours would have strengthened the study, since external validation of IOTA ADNEX showed it only performed moderately well in distinguishing between borderline or stage I tumours and more advanced cancers.”


