IgA mediated nephropathy is the world’s most common cause of glomerulonephritis, write Drs Bhadran Bose and Joomann Park
IgA (immunoglobulin A) nephropathy (IgAN) can affect patients of any age group but peak incidence is seen in the second and third decades of life. Varying male-to-female ratio is seen depending on region. It is rare in African-Americans but more common in East Asians and Caucasians.2
Patients can present with microscopic haematuria to fulminant renal failure. The main approach to treatment includes control of blood pressure and reducing proteinuria and in some selected cases immunosuppression.
Up to 25% of patients can develop end-stage renal disease.3
PATHOPHYSIOLOGY
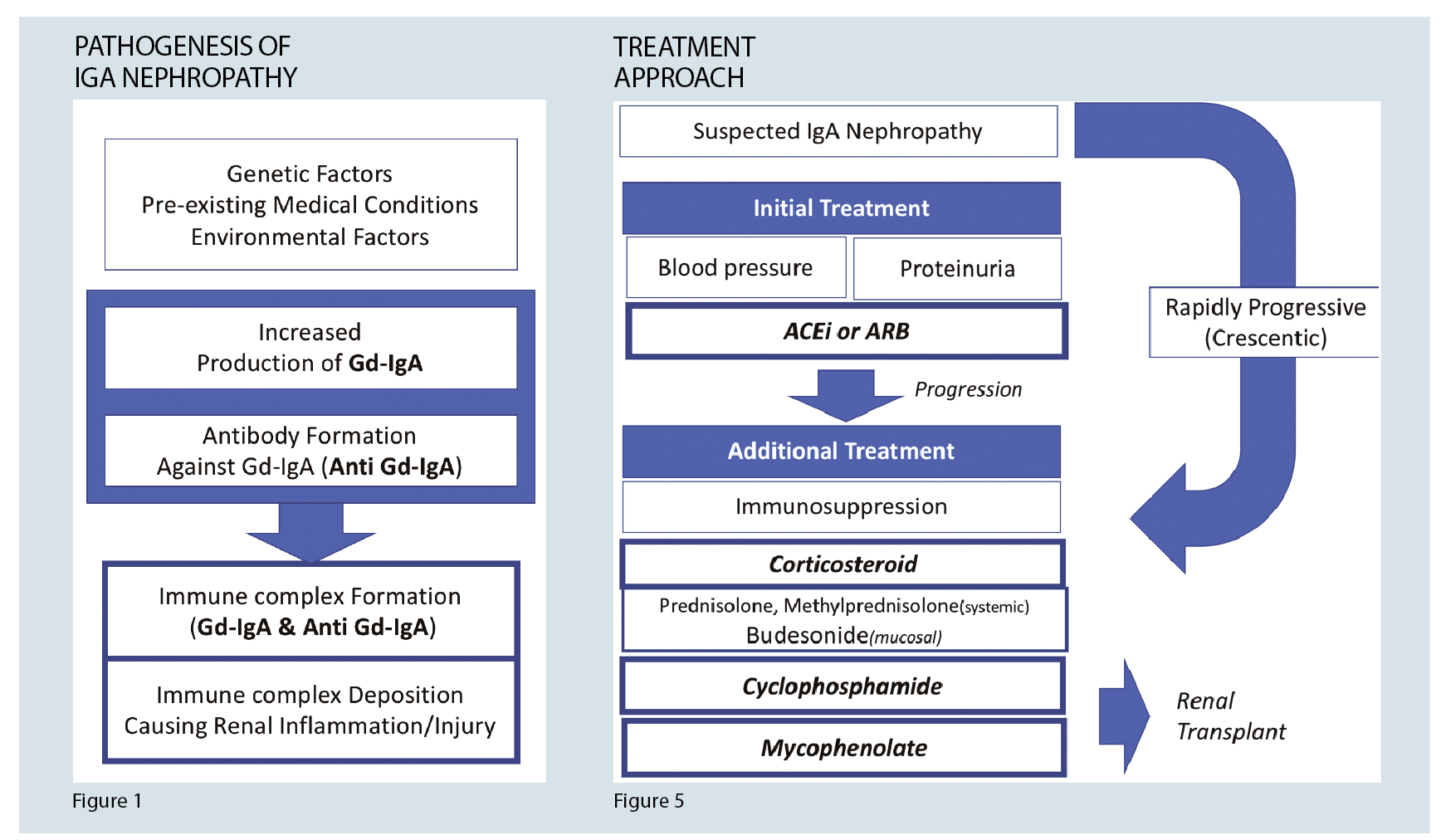
In IgA nephropathy, the primary cause for glomerular injury is an immune complex deposition in the mesangium. There is an increased amount of genetically determined circulating levels of IgA with galactose-deficient O-glycans and antibodies formed against these galactose deficient IgA. These complexes then accumulate in the glomerular mesangium. This process activates the mesangial cells, inducing proliferation and secretion of extracellular matrix, cytokines, and chemokines, which result in renal injury. 4 (See figure 1)
Viruses and bacteria have a similar antigen to galactose deficient IgA and this cross-reactivity may explain why IgA nephropathy is often associated with an infective episode.4
There is a familial component to this as well with family members often having elevated levels of circulating glycogen deficient IgA. The formation of antibody and antigen complexes precipitate this disease as the complexes cannot be cleared by the IgA catabolic pathway in the liver and hence deposit in the kidney.4
CLINICAL PRESENTATION
Close to half of the patients with IgA nephropathy present with a preceding upper respiratory tract infection and haematuria. Other accompanying symptoms include flank pain and low-grade fever. In older patients presenting with gross haematuria other diagnoses should also be considered such as malignancy.4
IgA nephropathy is often confused with post-streptococcal or post-infectious glomerulonephritis. However, this glomerulonephritis has an entirely different clinical course with a distinctive histological pattern. In such cases, timing may be helpful. Post-infectious glomerulonephritis tends to present four to six weeks after an infection.2
The second most common presentation is through microscopic haematuria and mild proteinuria found during routine/immigration medical examinations. These patients are generally otherwise asymptomatic.
The remaining 10% present with more severe and rapid symptoms/signs including nephrotic syndrome, rapidly progressive glomerulonephritis or acute renal insufficiency.
Henoch-Schönlein Purpura or IgA vasculitis can present with renal failure in one third of patients. IgA vasculitis shows identical histological changes to IgA nephropathy and similarly increased serum levels of glycogen deficienct IgA.5 While Henoch Scholein Purpura is more common in paediatric patients, adult patients with it are more likely to progress to end stage renal disease.6
IgA nehropathy can also occur in patients with severe liver disease, coeliac disease, HIV infection, minimal change disease and granulomatosis with polyangiitis.2 Therefore, it becomes important to screen for these conditions in patients with IgA nephropathy.
INVESTIGATIONS
There is no specific serological test for IgA nephropathy currently and renal biopsy is the gold standard in diagnosis. Urea, creatinine, urine analysis for haematuria and quantification of urine protein are routinely done to establish severity of disease.
Hepatitis B and C and HIV serology is useful to exclude conditions associated with the disease. Limited autoimmune screening may be beneficial in excluding systemic lupus erythematosus. Anti-streptolysin titre may be useful in excluding post-strep glomerulonephritis in certain presentations.
There are multiple tests under development to aid in the diagnosis and prognostication of IgA nephropathy. Anti-Gd-IgA IgG (Antibody) level and urinary proteomic analysis have been shown to have a high correlation with proteinuria and ACE inhibitor therapy responsiveness respectively.4
Ultrasound imaging is useful to exclude any underlying obstructive uropathy, congenital malformations and sometimes to assess chronicity of renal disease.
Biopsy is usually reserved for patients with rapidly progressive disease or severe disease as indicated by proteinuria (equal or more than 0.5g/day) and/or elevated creatinine. Patients with such features are at a higher risk of progression and therefore biopsy is warranted to guide aggressive therapy such as immunosuppression.7
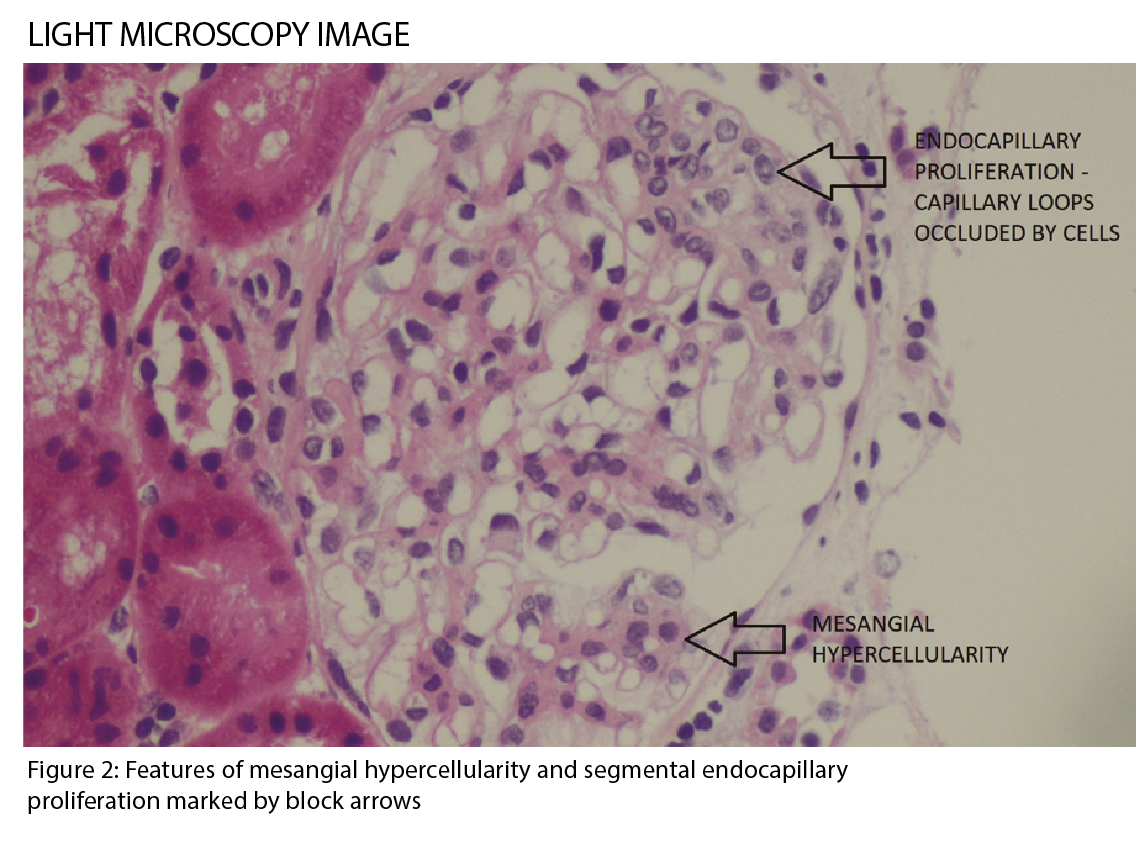
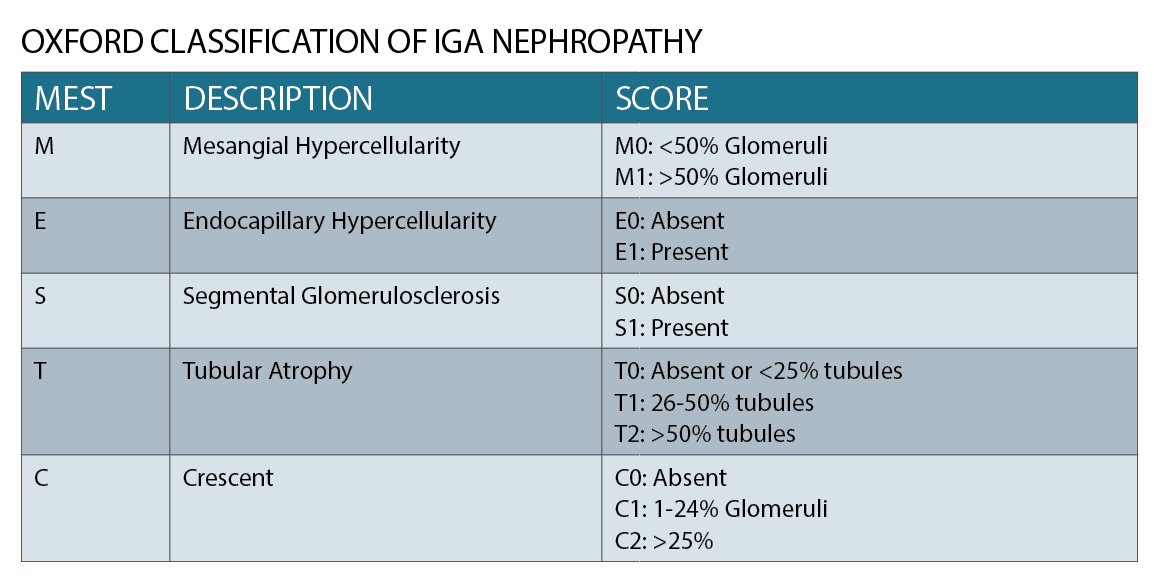
IgA nephropathy has characteristic histological features on microscopy (See figure 2), which forms part of the Oxford classification or MEST criteria. M refers to mesangial hypercellularity, E to endocapillary proliferation, S to segmental glomerulosclerosis and T to tubular atrophy. Each criterion has prognostic and management implications based on scoring.1 (See table)
Immunofluorescence staining shows diffuse mesangial uptake of IgA and C3. (See figure 3) Electron microscopy shows electron dense deposits in the mesangial or paramesangial region (See figure 4)4
MANAGEMENT
General approach
Patients with minor urine abnormalities, normal renal function and normal blood pressure do not need any intervention apart from biennial monitoring of renal function including urine for proteinuria. However, patients with significant amount of proteinuria (equal or more than 0.5g/day) need intervention as they are at a higher risk of progressing to end stage kidney disease.
Conservative therapy
Renin-angiotensin-aldosterone axis (RAS) blockade
The role of RAS blockade in IgA nephropathy is now well established. In a large meta-analysis of 585 patients, RAS blockade was shown to provide significant renal protection and reduction in proteinuria.8 However, dual blockade with a combination of angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blocker (ARB) is not recommended due to risks of hyperkalaemia.9
Target blood pressure varies depending on the level of proteinuria. Systolic of 130 and 120 is recommended for patients with proteinuria less than and more than 1g respectively.10
Fish oil
Fish oil treatment for IgA nephropathy has shown conflicting results. A Mayo clinic study showed that 12grams per day of fish oil prevented renal function decline in the long term, however no significant differences in blood pressure and proteinuria was noted.11 Overall, fish oil is commonly used as an adjunct given its relatively low side effect profile.
Other adjunctive therapies such as antiplatelet therapy and empirical tonsillectomy are not recommended based on evidence.10
Immunosuppressive therapy
Immunosuppressive therapy is used in rapidly progressing disease (crescents on biopsy) or failure to control disease with RAS blockade alone. KDIGO guidelines suggest consideration of immunosuppressive therapy if there is no improvement after 3-6 months of therapy, defined as ongoing proteinuria of ?1g day.10
Two recent major trials Stop IgAN13 and TESTING14 attempted to clarify the benefits and risks in immunosuppression.
Stop IgAN trial failed to demonstrate a significant difference in proteinuria or decline in eGFR in two immunosuppression arms (steroid and cyclophosphamide/azathioprine) despite a 35% increase in adverse events.
The TESTING trial showed that methylprednisolone use slowed eGFR decline and renal related death. Again though, the immunosuppression group had an unacceptably high adverse effects rate. Further trials are ongoing using a lower dose of corticosteroids.15
Cyclophosphamide with corticosteroids
Cyclophosphamide with corticosteroids is only considered in patients with rapidly progressive IgA nephropathy such as patients with crescentic features on histology. However, the benefit of this combination is very variable and it could depend on the racial background.
One study with Caucasian patients showed benefit16 whereas a study with Chinese patients did not.17
Mycophenolate
Mycophenolate had inconsistent results with some patients showing reduction in proteinuria and slowdown of decline in eGFR.18,19 There is a trial ongoing comparing prednisolone, mycophenolate and mycophenolate plus prednisolone.20
Rituximab
Rituximab did not demonstrate improved renal function or proteinuria in patients maintained on RAS blockade.21 Other immunosuppressive agents such as azathioprine, cyclosporine and tacrolimus have been investigated for treatment but without success.
Figure 5 summarises the treatment algorithm for IgA nephropathy.
Novel therapies
NEFIGAN trial investigated the use of novel targeted release formulation of oral budesonide. In this randomised control study, patients who received budesonide had a significant reduction in urine protein-creatinine ratio. The placebo group had an increase in urine protein-creatinine ratio.22
Sparsentan is a new agent with dual blockade of endothelin type A receptor and angiotensin receptor. In the phase 2 study (DUET), sparsentan demonstrated more proteinuria reduction compared to Irbesartan in patients with focal segmental glomerulosclerosis.23 Now there is a study looking at the use of sparsentan in IgA nephropathy.
There are clinical trials under way currently investigating the effect of proteasomal inhibitors, Blisibimod (selective peptibody antagonist of BAFF), spleen tyrosine kinase inhibition and Acthar gel in IgA nephropathy.
Despite treatment, if end-stage kidney disease develops, renal transplant is an appropriate option. IgA nephropathy may recur post-transplant. However, it still has the lowest mortality and allograft failure amongst transplant patients with primary glomerulonephritis.1
PROGNOSIS
Prognosis in IgA nephropathy is based on the Oxford classification and risk factors. Oxford classification is a histological grading system based on a case-controlled study with patients who have been diagnosed with the disease. The classification has been validated in both adult and children.
Proteinuria and hypertension have been shown to be major prognostic indicators in IgA nephropathy.4 Proteinuria has a linear correlation between renal outcomes even at lower levels.7 Patients with proteinuria of >3g/day lost renal function 24 times faster than those with proteinuria equal or less than 1g/day. Reduction in proteinuria following treatment also confers survival benefit irrespective of peak proteinuria after diagnosis.24
It is important to note that serum creatinine level is not a good marker for monitoring disease, eGFR may remain stable even with disease progression.25
Any patient suspected of IgA nephropathy should be referred to a renal physician for investigation and follow up management. An urgent referral is appropriate if the patient is showing severe hypertension and oliguria.
KDIGO guidelines recommend referral to a renal physician in the following circumstances: eGFR <30 or abrupt decline in eGFR, significant proteinuria, sustained urinary red cell casts and persistent hyperkalaemia.
SUMMARY
IgA nephropathy is a disease that affects both the young and old and is one of the most common causes of glomerular disease in Australia. It may present after an infection with haematuria, hypertension and/or deranged renal function.
There is no specific blood test to confirm IgA nephropathy. However, it may be suspected based on clinical history in the right demographic. Urinalysis and serology can provide information about severity of disease and associated conditions. Biopsy is the gold standard but mainly reserved for patients with heavy proteinuria.
Cornerstone of management is control of blood pressure and proteinuria with Renin-angiotensin-aldosterone axis blockade. Controlling proteinuria is essential as it has a prognostic significance. Immunosuppression may be indicated in rapidly progressing disease.
There is ongoing research to identify the best regimen to induce remission without severe side effects. New novel therapies such as oral budesonide and sparsentan are being currently investigated.
Dr Bhadran Bose is staff specialist in renal medicine at Nepean Hospital, Kingswood, New South Wales, and a VMO in Westmead Private and Norwest Private hospitals. He has a special interest in glomerular diseases and hypertensive disorders in pregnancy.
Dr Joomann Park is a medical registrar at Nepean Hospital, Kingswood, New South Wales.
Acknowledgement: The authors would like to thank Dr Jasveen Renthawa for providing the renal biopsy images.
References:
1. Soares MF, Roberts IS. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2017;26(3):165-71.
2. Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347(10):738-48.
3. Rekola S, Bergstrand A, Bucht H. Deterioration of GFR in IgA nephropathy as measured by 51Cr-EDTA clearance. Kidney Int. 1991;40(6):1050-4.
4. Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368(25):2402-14.
5. Kauffmann RH, Herrmann WA, Meyer CJ, Daha MR, Van Es LA. Circulating IgA-immune complexes in Henoch-Schonlein purpura. A longitudinal study of their relationship to disease activity and vascular deposition of IgA. Am J Med. 1980;69(6):859-66.
6. Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schonlein Purpura in adults: outcome and prognostic factors. J Am Soc Nephrol. 2002;13(5):1271-8.
7. Cattran DC, Reich HN, Beanlands HJ, Miller JA, Scholey JW, Troyanov S, et al. The impact of sex in primary glomerulonephritis. Nephrol Dial Transplant. 2008;23(7):2247-53.
8. Cheng J, Zhang W, Zhang XH, He Q, Tao XJ, Chen JH. ACEI/ARB therapy for IgA nephropathy: a meta analysis of randomised controlled trials. International journal of clinical practice. 2009;63(6):880-8.
9. Restriction of combined use of medicines affecting the renin-angiotensin system (RAS), (2014).
10. KDIGO. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3(1).
11. Donadio JV, Jr., Grande JP, Bergstralh EJ, Dart RA, Larson TS, Spencer DC. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. Mayo Nephrology Collaborative Group. J Am Soc Nephrol. 1999;10(8):1772-7.
12. Buemi M, Allegra A, Corica F, Aloisi C, Giacobbe M, Pettinato G, et al. Effect of fluvastatin on proteinuria in patients with immunoglobulin A nephropathy. Clinical pharmacology and therapeutics. 2000;67(4):427-31.
13. Makic MB, Rauen C, Jones K, Fisk AC. Continuing to challenge practice to be evidence based. Crit Care Nurse. 2015;35(2):39-50.
14. Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, et al. Effect of Oral Methylprednisolone on Clinical Outcomes in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA. 2017;318(5):432-42.
15. ClinicalTrials.gov. Therapeutic Evaluation of Steroids in IgA Nephropathy Global Study (TESTING Low Dose Study) (TESTING) 2018 [cited 2018 12 June 2018]. Available from: https://clinicaltrials.gov/ct2/show/NCT01560052.
16. Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002;13(1):142-8.
17. Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo Z, et al. Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. J Am Soc Nephrol. 2013;24(12):2118-25.
18. Chen X, Chen P, Cai G, Wu J, Cui Y, Zhang Y, et al. [A randomized control trial of mycophenolate mofeil treatment in severe IgA nephropathy]. Zhonghua Yi Xue Za Zhi. 2002;82(12):796-801.
19. Tang SC, Tang AW, Wong SS, Leung JC, Ho YW, Lai KN. Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int. 2010;77(6):543-9.
20. ClinicalTrials.gov. Mycophenolate Mofetil (MMF) in Patients With IgA Nephropathy (IgAN) 2018 [cited 2018 12 June 2018]. Available from: https://clinicaltrials.gov/ct2/show/record/NCT00657059.
21. Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, et al. A Randomized, Controlled Trial of Rituximab in IgA Nephropathy with Proteinuria and Renal Dysfunction. J Am Soc Nephrol. 2017;28(4):1306-13.
22. Fellstrom BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phasew 2b trial. Lancet. 2017;389(10084):2117-27.
23. Komers R, Gipson DS, Nelson P, Adler S, Srivastava T, Derebail VK, et al. Efficacy and Safety of Sparsentan Compared With Irbesartan in Patients With Primary Focal Segmental Glomerulosclerosis: Randomized, Controlled Trial Design (DUET). Kidney Int Rep. 2017;2(4):654-64.
24. Reich HN, Troyanov S, Scholey JW, Cattran DC, Toronto Glomerulonephritis R. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18(12):3177-83.
25. Alamartine E, Sabatier JC, Berthoux FC. Comparison of pathological lesions on repeated renal biopsies in 73 patients with primary IgA glomerulonephritis: value of quantitative scoring and approach to final prognosis. Clin Nephrol. 1990;34(2):45-51.