Some experts doubt it’ll ever be found. But that hasn’t dissuaded research groups from hunting for a universal flu vaccine
While flu shots are of fundamental importance to public health, the flu vaccine has always been the ugly duckling of the vaccine family. It is the only vaccine that needs to be updated every year, and its efficacy swings from about 20% to 60%.
Whereas viruses such as polio, rubella and measles have hardly mutated at all since vaccination programs began, the flu virus wears a new jacket of protein spikes every season, necessitating a new vaccine.
But it’s still the same virus. So there must be a way to get the immune system to see through that disguise – at least, that’s the fantasy that has gripped a generation of researchers searching for a universal flu vaccine.

Now, to mark the centenary of the deadly 1918 flu pandemic, the Bill and Melinda Gates Foundation want to realise this dream.
In April, Bill Gates announced a $12 million grant for universal flu vaccine research that was “off the beaten track” and “significantly radical in conception, and daring in premise”, with an eye to starting clinical trials by 2021.
A highly contagious, lethal, fast-moving airborne pathogen like the 1918 flu is one of the biggest pandemic threats we face.
That’s why we’ve launched a $12 million grand challenge to encourage novel approaches to a universal flu vaccine. https://t.co/CZm4WPRlNI
— Gates Foundation (@gatesfoundation) May 3, 2018
This funding comes at the tail end of a decades-long hunt for a universal flu vaccine, which has already yielded at least 10 separate Phase I trials, six Phase II trials, and 23 pre-clinical trials.
Professor Peter Palese, a microbiologist at the Icahn School of Medicine at Mount Sinai in New York, is among the closest to finding this “holy grail” of vaccinology.
His team have created a vaccine that redirects the immune response to the conserved regions of the flu virus, which are common across all strains. The vaccine gives broad protection against different influenza strains in mice and ferrets.
“It does work very well in mice against all the strains we tried,” Professor Palese told The Medical Republic. “We have selected probably 15 different strains and we were able to protect the mouse against every single virus challenge.”
Two separate Phase I human trials are now under way to test this vaccine as a live attenuated and inactivated form, supported by GSK and the Bill and Melinda Gates Foundation.
Even a universal flu vaccine that offered broad protection for a short time, say one to two years, could put a stop to a global pandemic.
But that’s “not good enough”, says Professor Palese. “I wouldn’t be happy with that. It should last a lifetime, or at least 20 years.”
A lot of hopes are pinned on the Palese strategy. He is reticent, however. “It ain’t over till it’s over. We really have to see if it works in people. Mouse experiments don’t tell you anything.”
How it works
The universal flu vaccine candidates under investigation all exploit the same feature of the flu virus: its tendency to conserve proteins.
The flu virus looks a bit like an echidna that has rolled itself into a ball, with spines shooting out on all sides.

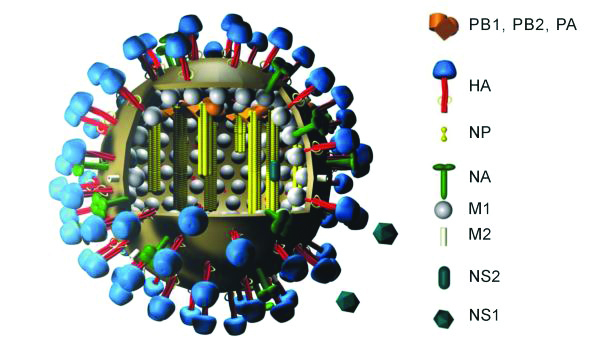
There are two types of spines, the proteins haemagglutinin (HA) and neuraminidase (NA).
When you look closely, HA is shaped somewhat like a mushroom, with a globular head and a stalk. The head region is notoriously variable; it changes with each season and differs across flu subtypes and lineages. The stalk region, however, remains fairly constant.
The HA head is readily accessible to the immune system (what scientists call “immunodominant”), so it is under strong selective pressure to evolve. HA head mutations have the potential to act like an invisibility cloak, allowing the virus to sneak past the immune system.
There is little survival value in the virus changing the structure of its HA stalk, however, so this region remains the same across all the different strains of flu.
A vaccine that redirects the immune response to the HA stalk region has the potential to be truly universal.
There are a few different ways to make this happen. Some research groups are making “headless” flu viruses, where only the HA stalk is present. Others have capped the HA heads to make the HA stalk more obvious.
Professor Palese’s team has replaced the normal HA head for an “exotic” HA head (a structure that exists in nature but is normally paired with a different HA stalk), with much success.

The team’s vaccine, which is currently being trialled in humans, is given as two doses: the first shot contains an artificial group A influenza virus with an “exotic” HA head, the second shot contains that same flu virus with a totally different HA head. By exposing the immune system repeatedly to constructs that express the same stalk domain and a different head domain, the vaccine stimulates a strong response to the conserved stalk region.
If the vaccine proves successful in humans, the researchers hope to roll it out as a trivalent vaccine, with each of the three main vaccine components having a different exotic head in each dose.
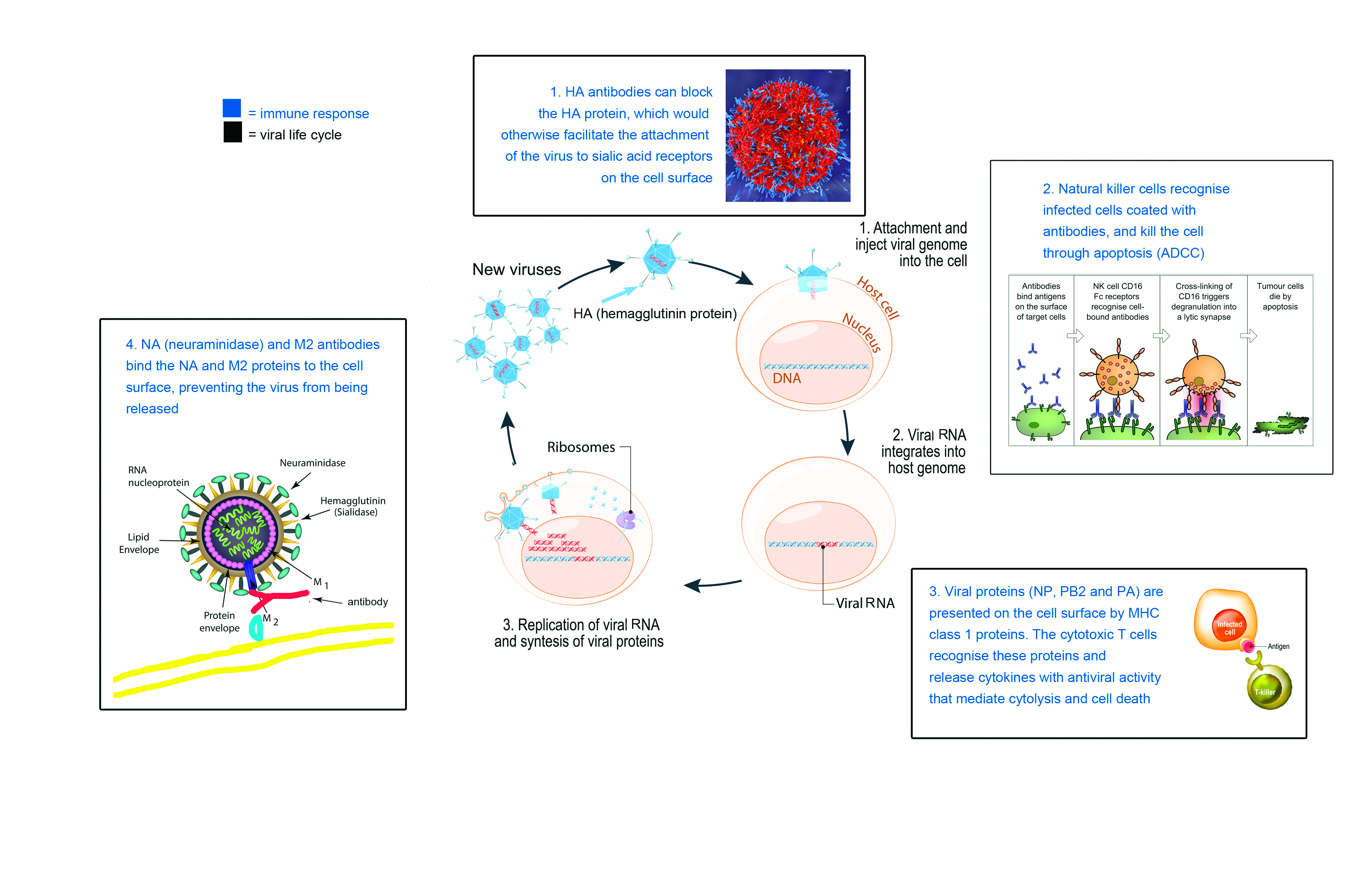
This vaccine triggers an immune response that stops the flu virus from getting into the cell. For this, it uses the same mechanism as conventional flu vaccines. When a traditional flu shot is injected into a person’s arm, the inactivated viral fragments stimulate the production of B cell antibodies, which block the HA protein.
The HA protein has one main job: to bind the flu virus to the cell membrane. Once inside the cell, the virus releases its RNA, hijacks the nucleus, and forces the cell machinery to do the work of replicating its genome.
“In conventional vaccines the antibody is actually made against the tip of the HA spike,” says Professor Palese. “If the antibody sits there, the virus can’t get in. In our case, we make antibodies against these conserved domains, [the HA stalk].”
Once the virus has replicated inside the cell, the NA protein (the second type of protein spike on the flu surface) acts as the getaway driver, helping to cleave the cell membrane and allowing the viruses to exit through budding.
Conventional vaccines do not build an immune response against the NA protein. But with Professor Palese’s vaccine, the HA head becomes immunosubdominant, thereby redirecting the immune system to the more conserved NA region.
The other thing that this universal flu vaccine does is wake up the cells involved in the humoral immune system’s second line of defence: natural killer cells. Natural killer cells play an analogous role to cytotoxic T cells in the adaptive immune system.
Infected cells are flagged for clearance through the attachment of specific antibodies. Natural killer cells bind to these antibodies via the Fc receptor, and release chemicals that cause the cell to burst and die. Fc receptors are a protein on the surface of the natural killer cell with binding specificity for the Fc (Fragment, crystallisable) region of the antibody.
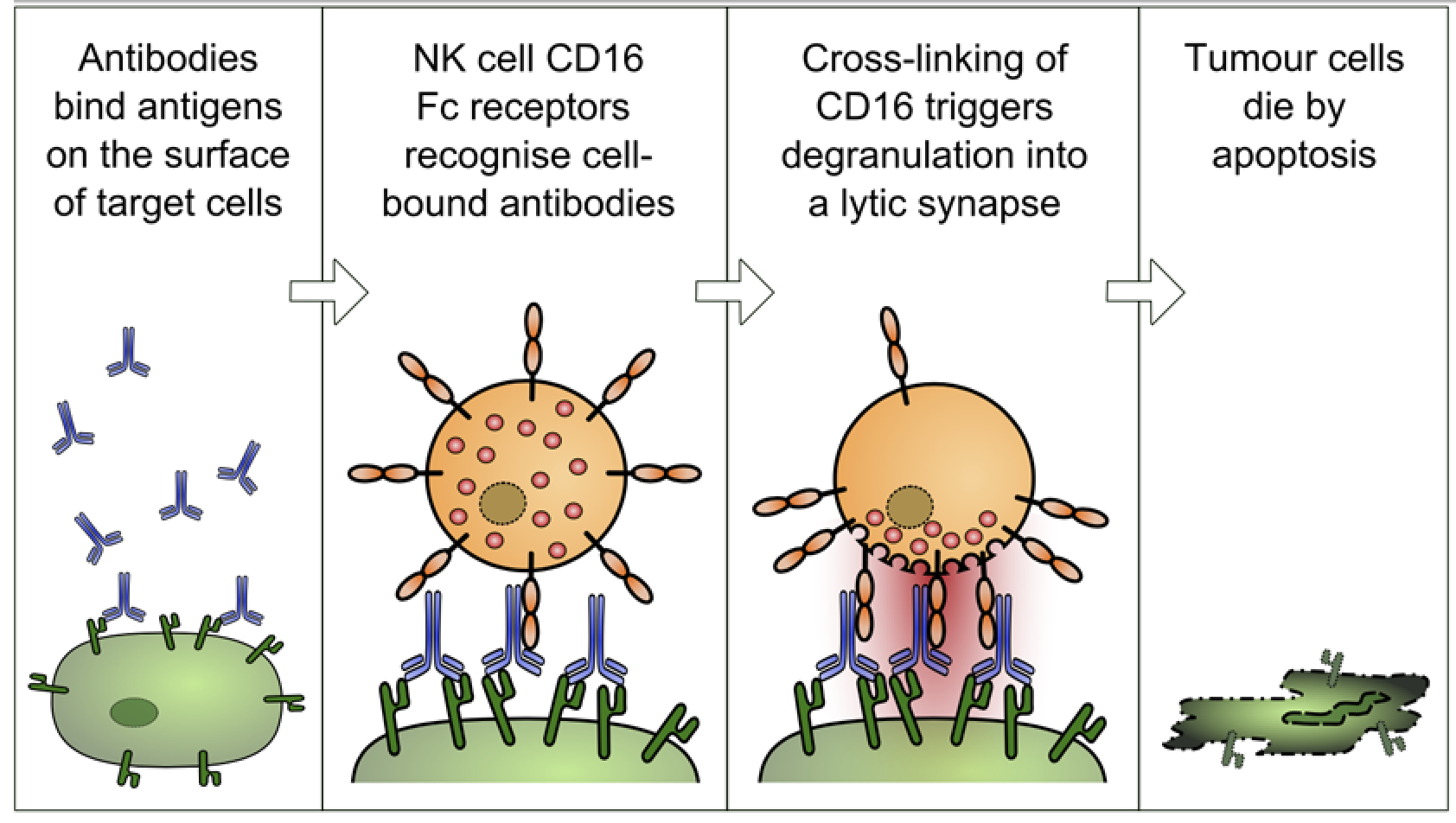
This mechanism, referred to as ADCC (antibody-dependent cell-mediated cytotoxicity), is the main way Professor Palese’s vaccine confers broad protection against many different flu virus strains.
Professor Palese’s vaccine appears to be very safe in mice and ferrets, but one published study hints at the possibility of adverse effects.
A single experiment using an adjuvanted vaccine in pigs, conducted by a group working at the FDA, showed an untoward effect in terms of autoimmunity.
But Professor Palese counters this study, pointing out that: “The immune system of a pig, particularly the lung, is very, very different from a human. It has 10 times more macrophages, for example.
“So this, I think, is a bad animal model, and they are also using a very outlandish adjuvant, which I think is probably causing that effect. We have studied many mice and ferrets and we have never seen this.”
Also, the antibody directed towards the stalk region of the HA is naturally produced in humans in low concentrations. If this antibody were associated with autoimmunity, we would have noticed, because people with higher levels of these antibodies would probably have autoimmune diseases.
The competition
The large spikes on the flu surface, HA and NA, are not the only proteins that have conserved regions across different flu strains. Another promising candidate for a universal flu vaccine is the matrix protein 2 ectodomain (M2e).
Research investigating the potential of M2e has been going on for a long time, but a paper published in Nature Communications this year by researchers at George State University in the US has given this old strategy a nanoparticle twist.
The team built a nanoparticle with M2e proteins at the core and a coating of headless HA proteins. In mice, this vaccine produced a “robust, long-lasting immunity, fully protecting the mice against challenges by divergent influenza A viruses”, the authors reported.
M2e plays an essential role in the virus lifecycle; it is a surface channel that opens once the virus is inside the cell, allowing proton flow across the viral membrane. This triggers the release of viral RNA into the cytoplasm, which then travels to the nucleus.
The human body has a natural antibody response to Me2 proteins, but it is pretty weak. “A possible explanation for this low immunogenicity is the small size of Me2 and the low abundance of M2 in virions compared to the large glycoproteins (HA and NA),” the authors write.
The human body produces antibodies that bind to M2e, preventing release of newly-replicated viruses into the extracellular fluid.
The researchers hope to enhance this natural antibody response by stuffing multiple copies of M2e into the nanoparticle vaccine.
Given how long researchers have been working on the M2e strategy, some experts are doubtful that this approach will ever progress into clinical trials. The M2e strategy has “never worked all that well, quite frankly”, says Professor Peter Doherty, a microbiologist at The Doherty Institute in Melbourne.
Triggering killer T cells
Many of the universal flu vaccine candidates that are furthest along in clinical trials rely on the adaptive immune system, namely killer T cells, to confer broad protection against multiple strains of flu.
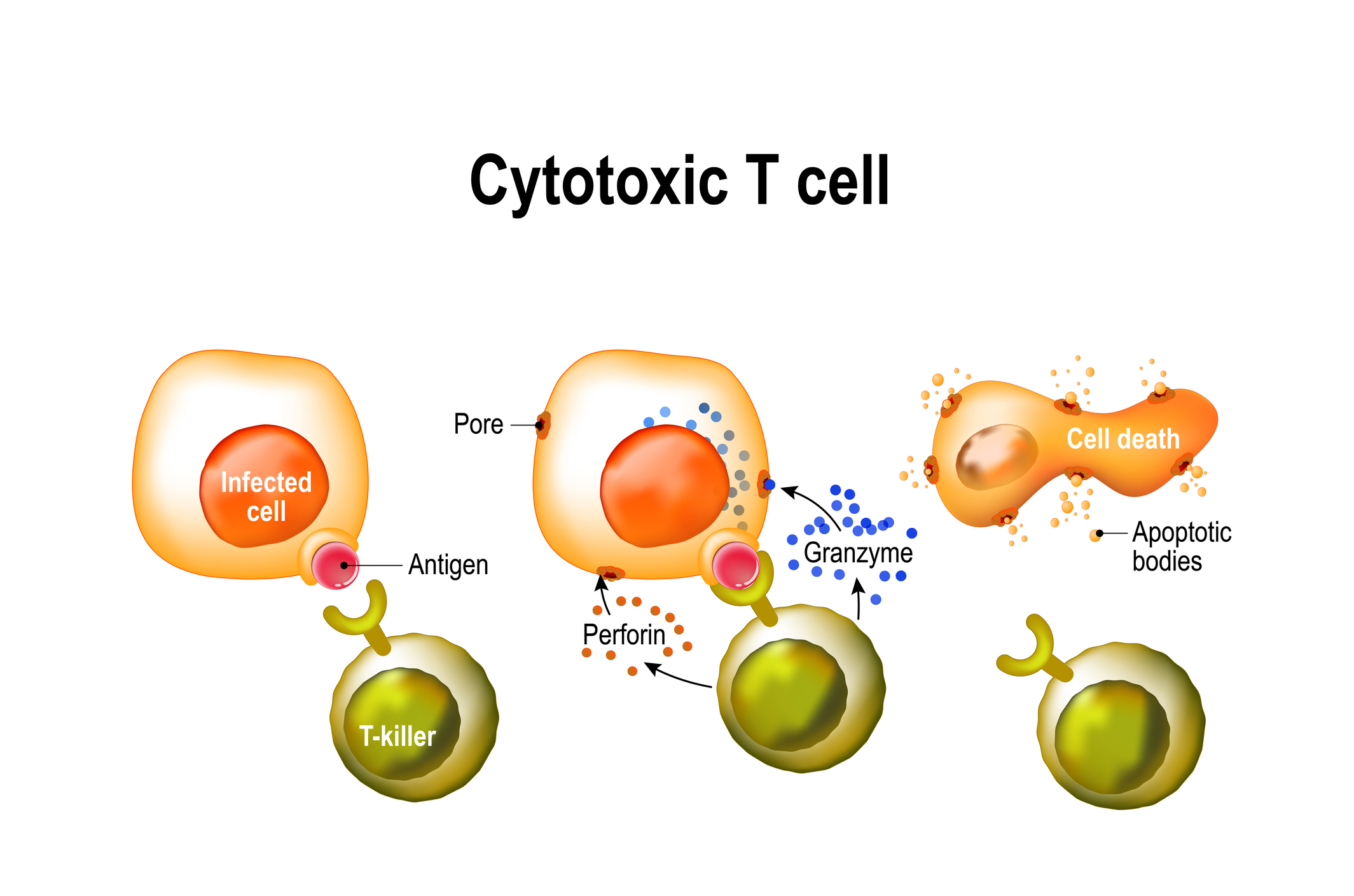
Cytotoxic T cells identify and kill infected cells that have ingested a virus, broken it down into individual parts, and displayed viral antigens on their cell surface.
Professor Doherty shared the 1996 Nobel Prize in medicine with scientist Rolf Zinkernagel for discovering how killer T cells recognise virus-infected cells. They found a set of cell surface proteins, called the major histocompatibility complex (MHC), that played a crucial role in the cell-mediated immune response.
Essentially, MHC molecules wave flu antigens around like miniature flags on the outside of an infected cell, telling the killer T cells to “come and get me”. By killing infected cells, T lymphocytes halt the production of viral replicates.
The antigens displayed by the MHC molecules tend to be the internal flu proteins, rather than the external HA proteins. These internal proteins are the same across all the different strains of flu, and can therefore form the basis of a universal flu vaccine.
But because T cells don’t prevent infection, and only slow the disease progression, “it would be a vaccine that decreased the severity of the disease but didn’t stop the disease,” says Professor Doherty.
Two companies with universal flu vaccines in phase II clinical trials are using this strategy.
Vaccitech, a spin-out company from the University of Oxford, has NHS funding to test its MVA+NP+M1 vaccine in more than 860 people over two years. This vaccine contains a viral vector, the modified vaccinia Ankara (MVA), which expresses the influenza A nucleoprotein (NP) and matrix protein 1 (M1).
BiondVax Pharmaceuticals’ vaccine Multimeric-001 is a single recombinant protein containing NP, M1, and conserved regions of the HA.
Both these vaccines direct the T cell memory toward internal and highly conserved influenza antigens, creating a broad heterosubtypic immunity.
The many faces of flu
The reason that we have waited so long for a universal flu vaccine is because the flu virus is a shape shifter.
There are up to 144 different subtypes in influenza group A, as well as multiple different lineages in group B and group C.
Influenza also has a large animal reservoir containing a host of different strains that have the potential to spill over into humans at any time, says Associate Professor Aeron Hurt, a scientist at a WHO Collaborating Centre for Reference and Research on Influenza. “And so the breadth of variation that exists between those viruses is such that to have one single vaccine that covers them all is very challenging,” he says.
Unlike other viruses, the flu doesn’t suffer a major fitness cost by mutating, says Professor Doherty.
Genetic mutations are much more frequent in flu than in other pathogens, such as bacteria, because the flu virus has no mechanism for fixing errors made during RNA replication.
The anatomy of the flu virus lends itself to modification. The spikes on the outside can morph into new forms without massively disrupting the functioning of the rest of the virus.
Making a vaccine that works despite the flu’s antigenic drifting and shifting is a major challenge. “The main problem is that the technology to do it properly just isn’t really there yet,” say Professor Doherty.
It’s all well and good to redirect the immune response to conserved regions of the flu virus, but there aren’t many of these sites, says Professor Hurt. “When we try to direct the immune response to these very conserved sites, the immune system tends to be swayed towards the less conserved sites,” he says.
Nightmare scenario
Waves of seasonal flu kill around half a million people worldwide each year. But the real terror comes from pandemic flu.
Every few decades, a subtype of influenza that is not normally found in humans jumps across from the animal reservoir of wild water birds, poultry and pigs.
The 1918 flu pandemic took about a year to reach every nook and cranny on Earth, eventually killing 50 million people. But travellers carried the swine flu around the world in a matter of weeks in 2009. This novel H1N1 strain was created through genetic reassortment (the swapping of RNA) between two different pig viruses.
And seasonal flu vaccines offer no protection during a global outbreak. An entirely new vaccine has to be produced to combat pandemic strains. But the production process is slow.
“We’re all frightened of a new, tremendously lethal pandemic virus,” says Professor Doherty.
“When the swine flu virus came, even though all the technology and everything was approved, it still took six months to get the vaccine out there.”
According to Professor Hurt, “The quickest you’d be looking at is three to four months after a pandemic before a vaccine would be available.
“And so during that time obviously there is a chance for a lot of cases of infection and a lot of deaths.”
Around 90% of vaccine production worldwide still depends on an egg-based process that hasn’t been updated in about 80 years.
There are only two flu vaccines on the US market that are not produced by converting hens’ eggs into miniature viral factories; a recombinant vaccine FluBlok, which uses an armyworm cell line to produce flu proteins expressed on a baculovirus vector, and a cell-based vaccine Flucelvax, which is grown in canine kidney cells.
The cells used to manufacture Flucelvax can be frozen and banked, allowing for a faster start-up of the manufacturing process in the event of a pandemic, according to the CDC. FluBlok production could also be scaled up quite quickly.
Ideally, we would have a universal flu vaccine as a backstop during emergencies. But that’s a bit of a pipe dream, says Professor Doherty.
“The basic story with the universal flu vaccine is it’s way off, if it’s ever a reality. Maybe recent articles put a better light on that. But there are quite a few people in the flu world that doubt that we will ever get a universal flu vaccine. I think it’s a good thing to keep exploring.”
What’s more likely to happen in the end is that we ramp up vaccine production to respond to a specific pandemic flu strain, he says.
“We are just going to get better at making a hell of a lot of stuff very fast.”