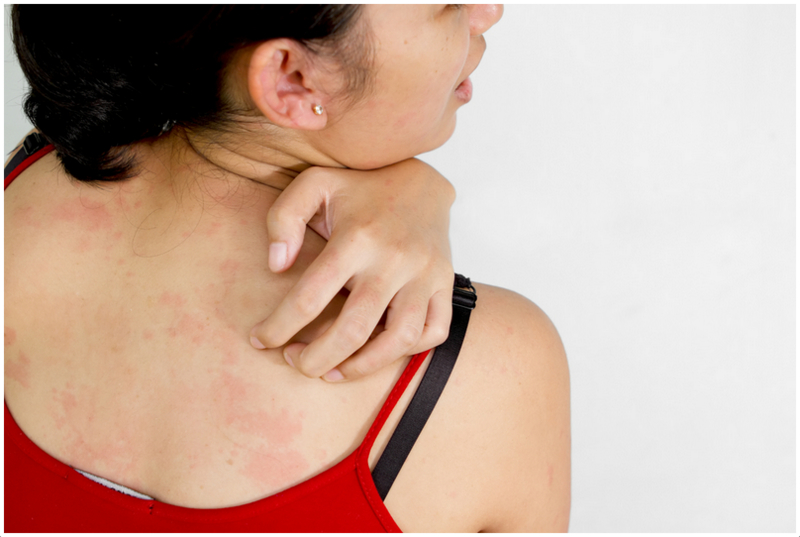
The PBS-listing for omalizumab has been extended to include patients with chronic spontaneous uticaria which is antihistamine-resistant
Patients with chronic spontaneous urticaria can now save up to $10,000 a year following a decision to PBS list the only drug available for its treatment.
GPs can now refer adults and adolescents over age-12 to a dermatologist or immunologist who will prescribe them omalizumab (Xolair, Novartis).
To be eligible for the treatment, patients need to be symptomatic despite standard therapy.
Head of immunology and allergy at Campbelltown Hospital, Professor Connie Katelaris, said there had been a significant treatment gap for patients with antihistamine-resistant chronic spontaneous urticaria.
While the mainstay treatment was antihistamine therapy, she said almost half of all patients were resistant to the treatment.
“Until now, these patients have been left with no viable long-term treatment option,” Professor Katelaris said.
Now, patients can affordably access a subcutaneous injection of humanised monoclonal antibody, that binds to human immunoglobulin E (IgE). This suppresses mast cell and basophil cell activation and inflammatory responses and can be used long-term in addition to H1 antihistamine therapy.
Studies suggest that a 12-week course can reduce itching by 71% compared with baseline, and almost completely resolve symptoms in almost half of patients.
Novartis recommends starting patients on two 150mg subcutaneous injections every four weeks, adding that some patients may only need one dose of 150mg every four weeks to achieve symptom control.
This listing follows a TGA approval for the drug for chronic spontaneous urticaria in December 2014, and a later PBS listing for children with severe allergic asthma late last year.
Common side effects of omalizumab include headache and nasopharyngitis.