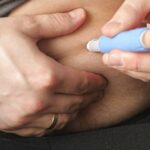
The TGA is warning prescribers not to start new patients on the GLP1-RA without a ‘compelling clinical reason’.
Supplies of the blockbuster semaglutide self-injectable Ozempic will be unable to meet demand for the foreseeable future, according to the Therapeutic Goods Administration, which says supplies should be conserved for patients already stabilised on the drug for whom no other options exist.
Maker Novo Nordisk has told the regulator that supply of the drug would be limited for the rest of this year and throughout 2024.
The TGA yesterday advised clinicians, after consulting with the Medicine Shortage Action Group, not to initiate new patients on Ozempic “unless there are no suitable alternatives or there is a compelling clinical reason to do so”.
“For patients who are already prescribed Ozempic, consider if they can be changed to an alternative (by consulting appropriate prescribing guidelines) as continuous supply cannot be guaranteed,” it said.
“Supplies should be conserved for patients who are stabilised on Ozempic who have no other treatment options. It is not known when the medicine will be available in sufficient quantities to meet the ongoing high demand.”
Ozempic, made sensationally popular by celebrity and influencer endorsements, is approved in Australia only for the management of type 2 diabetes, but is frequently prescribed off label for weight loss. The formulation sold as Wegovy, also made by Novo Nordisk, has been approved as a weight-loss therapy for patients meeting certain criteria, but has never been available in Australia.
A competitor self-injectable, tirzepatide (Mounjaro, Eli Lilly) has been approved by the TGA for poorly controlled type 2 diabetes, but rejected for a PBS listing.
Tirzepatide, a combined GLP-1 and GIP (glucose-dependent insulinotropic polypeptide) receptor agonist, is reported to have shown success in reducing Hba1C and body weight in adults, with good tolerability and a similar safety profile to the GLP-1 RAs semaglutide and dulaglutide.
Australian Diabetes Society CEO Associated Professor Sof Andrikopoulos told TMR last month that “the weight loss and then the subsequent reduction in HbA1c is better than what we’ve seen with any other drug”.
Eli Lilly said it hoped the product would be available privately in Australia by the end of this month, with associate vice president of medical Dr Kevin Lim saying: “We’ve been very careful about launching only when we know that we’re going to be providing enough supply.”