Widespread misinformation on the use of MHT has meant that up to two-thirds of women suffering severe post-menopausal issues will still not seek help - SPONSORED
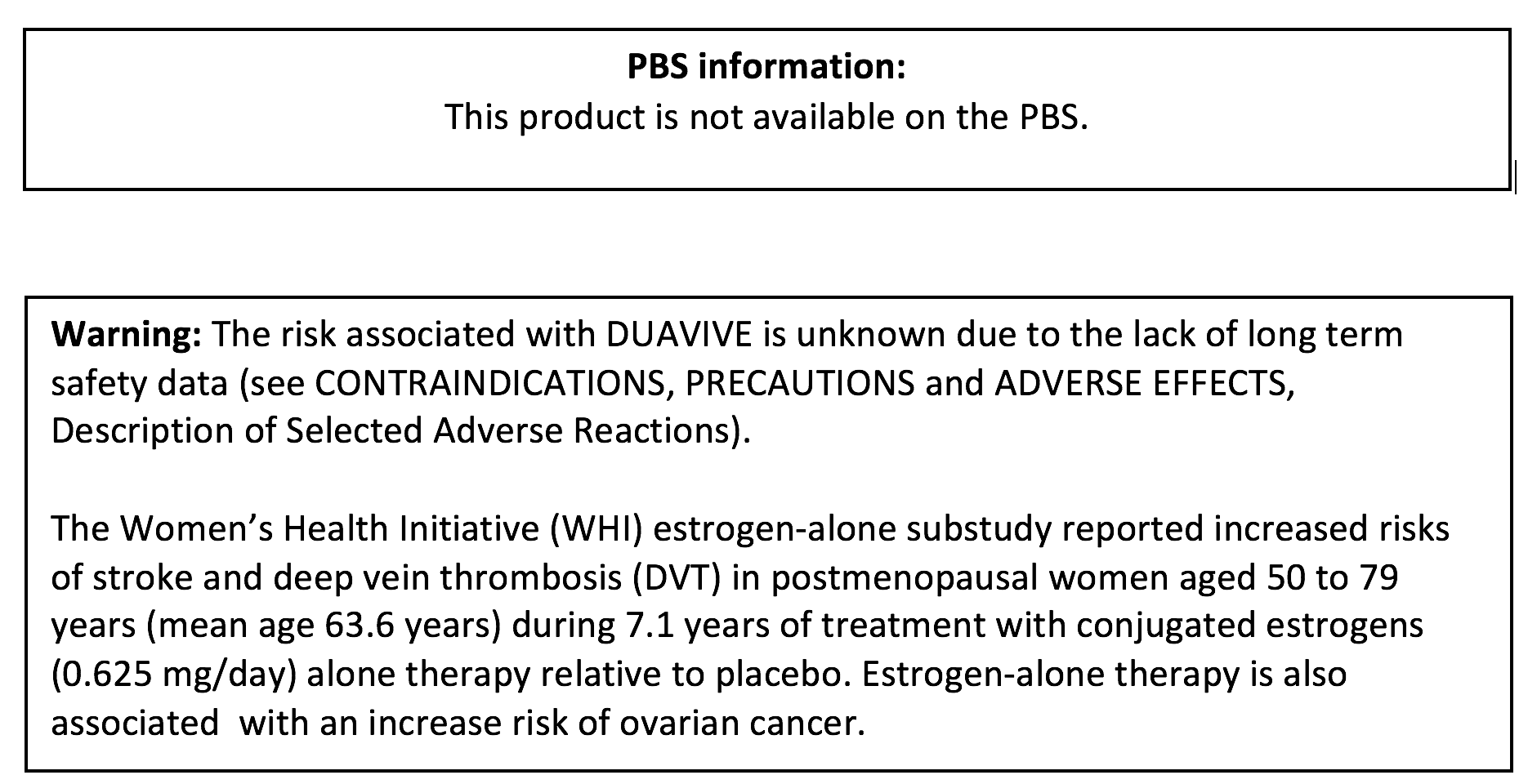
The findings of the 2002 Women’s Health Initiative (WHI) study, resulted in more than a decade of confusion when treating menopausal symptoms with menopause hormone therapy (MHT). Although valuable information was gained, inconsistent interpretation of the results led to widespread misinformation on the use of MHT. (1)
Since the initial publication of the study, much research and work has been done which should have restored the reputation of MHT, but the spectre of the WHI study, and ongoing incidence of issues associated largely with the use of progestin in combined estrogen and progestin therapy, has meant that up to two-thirds of women suffering severe post-menopausal issues will still not seek help (2,3).
In this short video, Professor Susan Davis, an NHMRC Principal Research Fellow and Chair of Women’s Health Research Program, discusses the next generation of MHT which involves the combination of estrogen with selective estrogen receptor modulator (SERM), as an alternative to progestin.
[media_embed]https://youtu.be/bzmcFhdf5hc[/media_embed]
This new generation therapy holds the hope and opportunity of encouraging a conversation about MHT, to help ensure the next generation of women seek and receive appropriate treatment for their symptoms.
Before Prescribing Duavive , please review the full Product Information at https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2016-PI-02879-1#dec
References:
1: Langer, RD. The evidence base for HRT: what can we believe? Climacteric, 2017 Vol. 20, No. 2, 91–96
2: HRT Benefits and Risks: What you should know, Women’s Health Concern Fact Sheet, British Menopause Society, December 2015
3: Beverley Lawton, et al, Changes in use of hormone replacement therapy after the report from the Women’s Health Initiative: cross sectional survey of users, BMJ 2003;327:845–6
PP-DUA-AUS-0224 10/17
© Pfizer 2017
Pfizer Australia Pty Limited. Pfizer Medical Information: 1800 675 229. 38–42 Wharf Road, West Ryde, NSW, 2114.