Elective surgery can resume, thanks to a fresh supply of protective and other equipment, and the government says Australia is in a position to begin a cautious recovery.
Thanks for joining us today on The Medical Republic‘s live COVID-19 blog.
And thank you to our sponsor and supporter for funding this project with an independent grant, Boehringer Ingelheim
The latest
- Are we going far enough with physical distancing? Maybe not, research suggests.
- New bulk-billing incentives for patients at risk of COVID-19.
- Elective surgery to resume with arrival of more protective and other equipment, Hunt says
- After some solid ploughing through the MBS Online website, Penny Durham is delivering a bit more clarity on the new item numbers and bulk-billing situation:
Specialists may now bill as usual for all patients they see by telehealth, now that the government has dropped the requirement that they bulk-bill those deemed vulnerable to COVID-19 – a list that includes anyone with a chronic condition.
GPs still need to bulk-bill children under 16 and concessional patients, as well as the vulnerable, who also include Indigenous patients over 50 and anyone else over 70, pregnant women, parents of new babies, and the immune-compromised.
All other patients can be billed at the GP’s discretion.
GPs can now also claim bulk-billing incentives of between $12.75 and $19.30 (numbers 10981 and 10982), for seeing vulnerable patients either by telehealth or face to face. These are in addition to the bulk-billing incentives for patients who are under 16 or concession holders (items 10990, 10991 and 10992).
A further 28 new telehealth items are available for specialists – consultant psychiatrists, public health physicians and neurosurgeons – and for services provided by a practice nurse or Aboriginal and Torres Strait Islander health practitioner on behalf of a medical practitioner.
- Physical distancing is based on assumptions of droplet spread from the 1930s, say the authors of a study which suggests respiratory droplets could carry well beyond two metres.
Australian and US researchers reviewed the evidence for the current guidance of 1-2 metres of physical distancing, which is incorporated into guidelines from organisations such as the WHO, and found it to be ‘sparse’.
Writing in The Journal of Infectious Diseases, the authors reported that eight of ten studies reviewed showed that droplets travelled horizontally more than two metres, and in some cases more than eight metres. Several studies also suggested aerosol transmission of SARS-CoV-2, with one recording the virus transmitted over four metres.
They suggested it was timely to review the evidence informing the guidelines on droplet precautions.
“This review reveals the limited scientific data to inform spatial separation guidelines, and a growing body of evidence that droplet precautions are not appropriate for SARS-CoV-2,” they wrote.
- Two new bulk-billing incentive items have been added to the Medicare Benefits Schedule for providing either face-to-face or telehealth medical services to a person who is at risk of COVID-19.
The two new numbers mirror the existing 10990 and 10991 item number, but the listings don’t specify what constitutes ‘at risk’ for COVID-19.
- Penny Durham reports: Elective surgery can resume, thanks to a fresh supply of protective and other equipment, and the government says Australia is in a position to begin a cautious recovery.
Health Minister Greg Hunt said this afternoon that 60 million new masks had arrived in the country, with 22 million already distributed and 11.5 million more to be distributed this week. A further 100 million masks had been secured and would arrive over the next six weeks.
Categories 1, 2 and urgent category 3 surgeries could therefore begin again after this weekend.
Mr Hunt said 3260 new ventilators had been delivered to hospitals – 3000 non-invasive and 260 invasive – bringing our capacity to 7500.
He said Australia had seen a “sustained and consolidated flattening of the curve” with a less than 1% increase for nine consecutive days.
For fear of a second wave of infections like that seen in Singapore, restrictions on social and commercial activities will not be lifted quickly, and travel bans in and out of Australia have been extended.
Prime Minister Scott Morrison said people were mistaken about two of the restrictions in place, believing that visits to aged care were banned – they are limited to two a day and with appropriate screening – and that schools were obliged to operate with 4m2 per person, when in fact they are asked to have smaller class sizes.
He said the data collected by the proposed contact tracing app would be inaccessible to any federal government agency. Access would only be available to the state health department officials who were engaged in tracing contacts of known cases. He said it was a “more comprehensive and foolproof system” than the diary-keeping approach being tried in New Zealand.
- TMR’s Penny Durham writes: We’ve just had a briefing on the AustralaSian COVID-19 Trial (ASCOT), led by Associate Professor Steven Tong from the Doherty Institute and Royal Melbourne Hospital, which will test hospital patients on the drugs lopinavir/ritonavir (Kaletra) and hydroxychloroquine.
The study aims to recruit more than 2000 patients from 70 Australian hospitals and 11 New Zealand hospitals – with overseas cohorts as well if they don’t get the numbers – and will measure whether either drug or a combination can prevent mild cases from progressing to the point of requiring ICU treatment or ventilation.
Doherty director Professor Sharon Lewin said the drugs had definitely shown antiviral activity, but only in vitro so far.
The trial is expected to run 12-18 months and has a responsive-adaptive design, so dosages can be dialled up or down according to early results, and further treatments could be added to the protocol.
It was open-label, said Professor David Paterson, director of University of Queensland’s Centre for Clinical Research, because it was impractical to take the time to design a matched placebo and the outcomes were objective enough that blinding was not crucial.
Professor Joshua Davis, president of the Australasian Society for Infectious Diseases, said the trial would be so large and distributed that all non-ICU patients across the two countries should have the chance to participate. He said the study would complement others in Australia and overseas, with their measures harmonised so the data could be combined.
Professor Tong said the trial had been planned to test hydroxychloroquine before Donald Trump created a global wave of hype, drawing on a small and weakly designed French study led by Professor Didier Raoult.
Professor Paterson said: “The reason we’re doing a large randomised trial is to tease out all the speculation and confusing information from smaller studies such as the ones out of France and Brazil.
“Being able to recruit more than 2000 patients should allow us to be definitive on whether it works and what the safety profile is for patients with COVID-19.”
Dr Susan Morpeth from the University of Auckland said data would be collected on Maori and Pacific populations, who were regarded as highly susceptible to the virus.
It was reported today that Gilead Sciences, which makes the antiviral remdesivir, had denied ASCOT a supply of the drug.
The researchers said that short supply had meant the company wanted to keep stocks in countries with more cases, but the adaptive design of ASCOT meant that remdesivir could be included if more supplies became available.
- Got some views on how the Australian government has handled the COVID-19 pandemic, finely honed by fighting with idiots on social media or arguing with family members?
Now’s your chance to bend the ear of those in charge. Tell ‘em what you really think and the best part is, they can’t argue back.
The Senate’s committee on COVID-19 is calling for submissions to its inquiry on the government’s response to the pandemic. The closing date for submissions is 28 May 2020, and the final report is expected to drop on or before 30 June 2022.
We’ll get the popcorn ready.
- New telehealth MBS item numbers for practice nurses and Aboriginal and Torres Strait Islander health practitioners are now available, to enable at-home care for people with chronic conditions.
The Australian Primary Health Care Nurses Association welcomed the announcement, having campaigned for practice nurses to be included in telehealth to help keep people with complex conditions away from hospitals during the COVID-19 pandemic.
The four new item numbers mirror existing MBS item numbers 10987 and 10997.
The government also announced 24 new telehealth item numbers for specialists; six for consultant psychiatrists, eight for public health physicians and 10 for neurosurgeons.
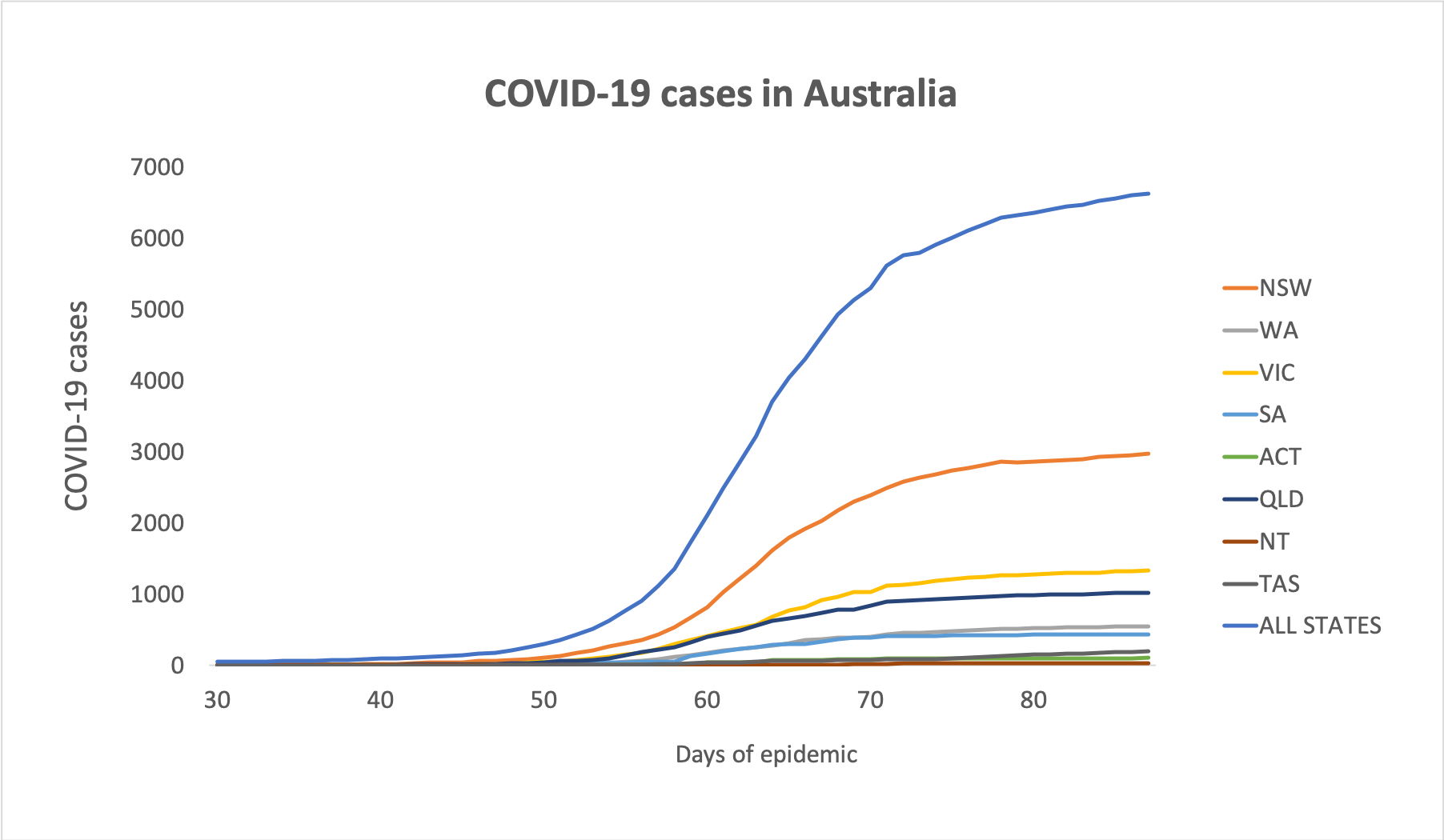
The bulk-billing requirement for COVID-19 telehealth has been lifted for specialists and allied health providers, who may now continue their usual billing practices. - Here are the latest figures on confirmed COVID-19 infections around Australia, to 3pm yesterday:
National – 6,625 (up 13) and 71 deaths
ACT – 104
NSW – 2969
NT – 27
QLD – 1019
SA – 435
TAS – 197
VIC – 1329
WA – 545
Disclaimer: The content on the Medical Republic COVID-19 blog is independently created by Medical Republic without input from Boehringer Ingelheim Pty Ltd. The views, information, or opinions expressed on the Medical Republic COVID-19 blog are Medical Republic’s own and do not necessarily represent those of Boehringer Ingelheim Pty Ltd. Boehringer Ingelheim Pty Ltd is not responsible for and does not verify the accuracy of any content on the Medical Republic COVID-19 blog.


