Remdesivir has fallen out of favour, with the World Health Organisation now recommending against its use in patients with confirmed COVID-19, regardless of disease severity.
Welcome to The Medical Republic’s COVID Catch-Up.
It’s the day’s COVID-19 news in one convenient post. Email bianca@biancanogrady.com with any tips, comments or feedback.
20 November
- The WHO recommends against using remdesivir for COVID-19.
- While viral shedding lasts for weeks, live SARS-CoV-2 only hangs around for nine days, studies suggest.
- Oxford vaccine well tolerated and generates neutralising antibodies in older adults.
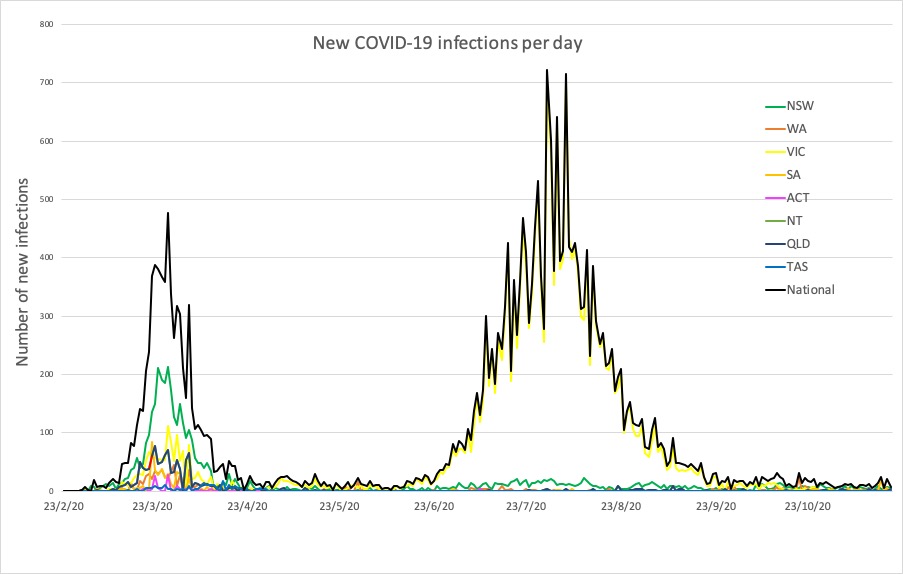
- South Australia unlocks, and the latest confirmed COVID-19 infection numbers from around Australia.
- Remdesivir has fallen out of favour, with the World Health Organisation now recommending against its use in patients with confirmed COVID-19, regardless of disease severity.
In updating its living guidelines on management of COVID-19, WHO noted that the evidence suggested any beneficial effects of remdesivir were likely to be small, the possibility of harm remains, and that most patients would likely not prefer an intravenous therapy based on such low certainty evidence. - People infected with SARS-CoV-2 shed viral RNA for an average of 17 days after infection but seem unlikely to shed live virus beyond nine days after infection, a systematic review and meta-analysis has found.
The paper, published in The Lancet Microbe, examined 79 studies – involving 5340 individuals – of the viral dynamics of SARS-CoV-2. This revealed that viral RNA could be found in the upper respiratory tract up to 83 days after infection, and in stool samples up to 126 days after infection.
Viral loads were highest at or just after the time of symptom onset, or 3-5 days after. However even in individuals with persistently high viral loads, no live virus could be isolated more than nine days after the onset of symptoms.
The studies also suggested that asymptomatic individuals had similar or slightly lower viral loads than symptomatic individuals, but a much shorter duration of viral shedding. - The Oxford vaccine appears to be well-tolerated by older adults and achieve similar antibody responses to those seen in younger individuals, according to a paper in the Lancet.
Results from the single-blind, multicentre, randomised, controlled, phase 2 trial of the two-dose vaccine – which has involved 560 participants including 240 aged 70 years or older – showed similar levels of neutralising antibodies in all individuals who had received both doses, regardless of age.
The study results also suggested that older adults tolerated the vaccine better than younger, with both local and systemic reactions less common in those aged over 65 years. - South Australians are wrestling with relief and rage after learning that the state’s circuit-breaking six-day lockdown was triggered by a lie told to contact tracers.
According to The Guardian, an individual who had tested positive for the virus claimed they had only purchased a pizza from a pizza outlet, when in fact they worked there. The lie gave the impression that the virus was much more fast-moving, which prompted the swift intervention to keep it in check.
While Premier Steven Marshall stressed that the Parafield cluster was still a serious health concern, he announced that the lockdown would lift sooner than planned. Families are now permitted to exercise together, and from midnight on Saturday, the stay-at-home order will lift. New public health measures will be announced, limiting density at public locations and events, but schools will also reopen.
Here are the latest confirmed COVID-19 infection numbers from around Australia to 9pm Thursday:
National – 27,784, with 907 deaths
ACT – 115 (0)
NSW – 4514 (5)
NT – 46 (0)
QLD – 1190 (3)
SA – 550 (0)
TAS – 230 (0)
VIC – 20,345 (0)
WA – 794 (0)