Children may develop antibodies to SARS-CoV-2 and symptoms of COVID-19 without ever testing positive to the virus using RT-PCR, research suggests.
Welcome to The Medical Republic’s COVID Catch-Up.
It’s the day’s COVID-19 news in one convenient post. Email bianca@biancanogrady.com with any tips, comments or feedback.
12 November
- Children can be symptomatic and have SARS-CoV-2 antibodies but still test negative on RT-PCR.
- North Dakota asks asymptomatic, COVID-19-positive nurses to keep working.
- Vaccines usually take eight years to get to FDA approval, study finds.
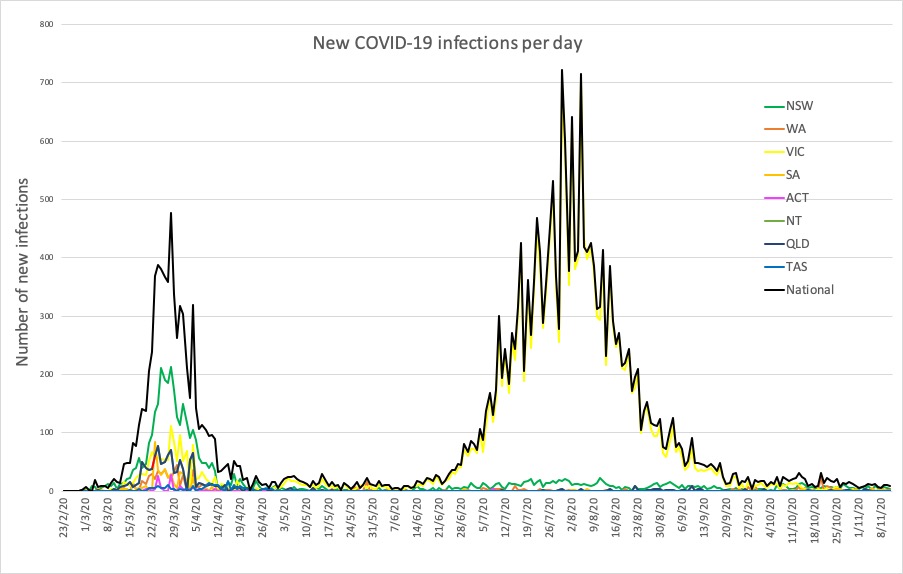
- Latest confirmed COVID-19 infections from around Australia.
- Children may develop antibodies to SARS-CoV-2 and symptoms of COVID-19 without ever testing positive to the virus using RT-PCR, research suggests.
An Australian case study reported in Nature Communications described a Melbourne family of two adults – both with PCR-confirmed symptomatic SARS-CoV-2 infection – and three children aged five, seven and nine years, who were followed for 88 days with samples taken every few days.
The children consistently tested negative for SARS-CoV-2 on RT-PCR despite close contact in the household, including one child sleeping in the same bed as the parents. Two of the children developed symptoms including runny nose, cough, abdominal pain and diarrhoea but the third was asymptomatic.
All five family members showed a cellular immune response consistent with infection, as well as testing positive for antibodies against SARS-CoV-2 in their saliva and sera. Both parents and one child also showed varying levels of neutralising antibodies against SARS-CoV-2.
Interestingly, the child who remained asymptomatic throughout was the one with the strongest antibody response to the virus.
The authors commented that the fact the children had similar cellular and antibody-based responses suggests they were infected but were able to fend off the virus more effectively than their parents. However, this doesn’t answer the question of whether they will be protected from reinfection. - Things are so bad in the US state of North Dakota that the governor has announced that asymptomatic SARS-CoV-2-positive nurses can continue to work in healthcare facilities.
As astonishing as this scenario is, it’s one that the US Centers for Disease Control considered back in July and even provided guidelines for.
The guidelines say these infected staff should be prioritised for jobs that don’t involve contact with patients or other staff, such as telemedicine, or to interact only with patients who are also SARS-CoV-2-positive. But there is even the last-resort option of having these nurses care for patients who do not have COVID-19.
A report in the Grand Forks Herald newspaper said the state’s hospitals are nearing capacity, with a lack of beds and major staff shortages. Case numbers in North Dakota have increased by nearly 19% in the past seven days. More than a thousand cases have been reported in the past 24 hours alone, in a state with a population of 762,000. - Vaccines typically take eight years to get from the first stage of clinical development to approval by the US Food and Drug Administration, and the FDA usually takes around one year to review and approve a vaccine, a study has found.
To put that into perspective, the first cases of SARS-CoV-2 were reported by Chinese officials in early December 2019 and the full genetic sequence of the virus wasn’t published until January 2020.
The study, published in JAMA Internal Medicine, found each vaccine approval was supported by a median of seven clinical trials, tested in a median of 6710 patients, and with a median follow-up of six months.
The FDA has not yet approved any SARS-CoV-2 vaccines, although there are ten vaccine candidates currently in phase 3 clinical trials around the world, including one involving researchers at the Peter Doherty Institute.
Meanwhile, Russia has announced that its Sputnik V vaccine shows 92% efficacy, after 16,000 volunteers have received two doses of the vaccine and been followed for 21 days after the first injection.
The press release was as light on details as Pfizer’s announcement earlier this week. Only 20 cases of SARS-CoV-2 infection have been detected in the study populations so far, but the release provided no information about the infection rate breakdown between the vaccine and placebo groups, or the effect in subgroups such as older people.
Researchers reported no ‘unexpected’ adverse events other than short-term side effects such as pain at the injection site and flu-like syndrome. - Sewage testing has picked up fragments of SARS-CoV-2 RNA at two locations in western Sydney, prompting NSW Health to call for all residents with even the mildest of symptoms to get tested for COVID-19.
The areas serviced by the pumping stations are North Kellyville, Rouse Hill, Box Hill, The Ponds, Kellyville Ridge, Parklea, Quakers Hill and Acacia Gardens.
Meanwhile, NSW reported four new cases yesterday but all in returned travellers in hotel quarantine. Western Australia’s two new cases are also returned travellers in hotel quarantine.
South Australia has reported a case in a woman who had previously tested positive in Victoria, then apparently cleared the virus, but tested positive again and is now isolating in a medi-hotel.
Here are today’s confirmed COVID-19 infection numbers from around Australia to 9pm Wednesday:
National – 27,686, with 907 deaths
ACT – 114 (0)
NSW – 4478 (4)
NT – 41 (0)
QLD – 1179 (1)
SA – 519 (1)
TAS – 230 (0)
VIC – 20,345 (0)
WA – 780 (2)