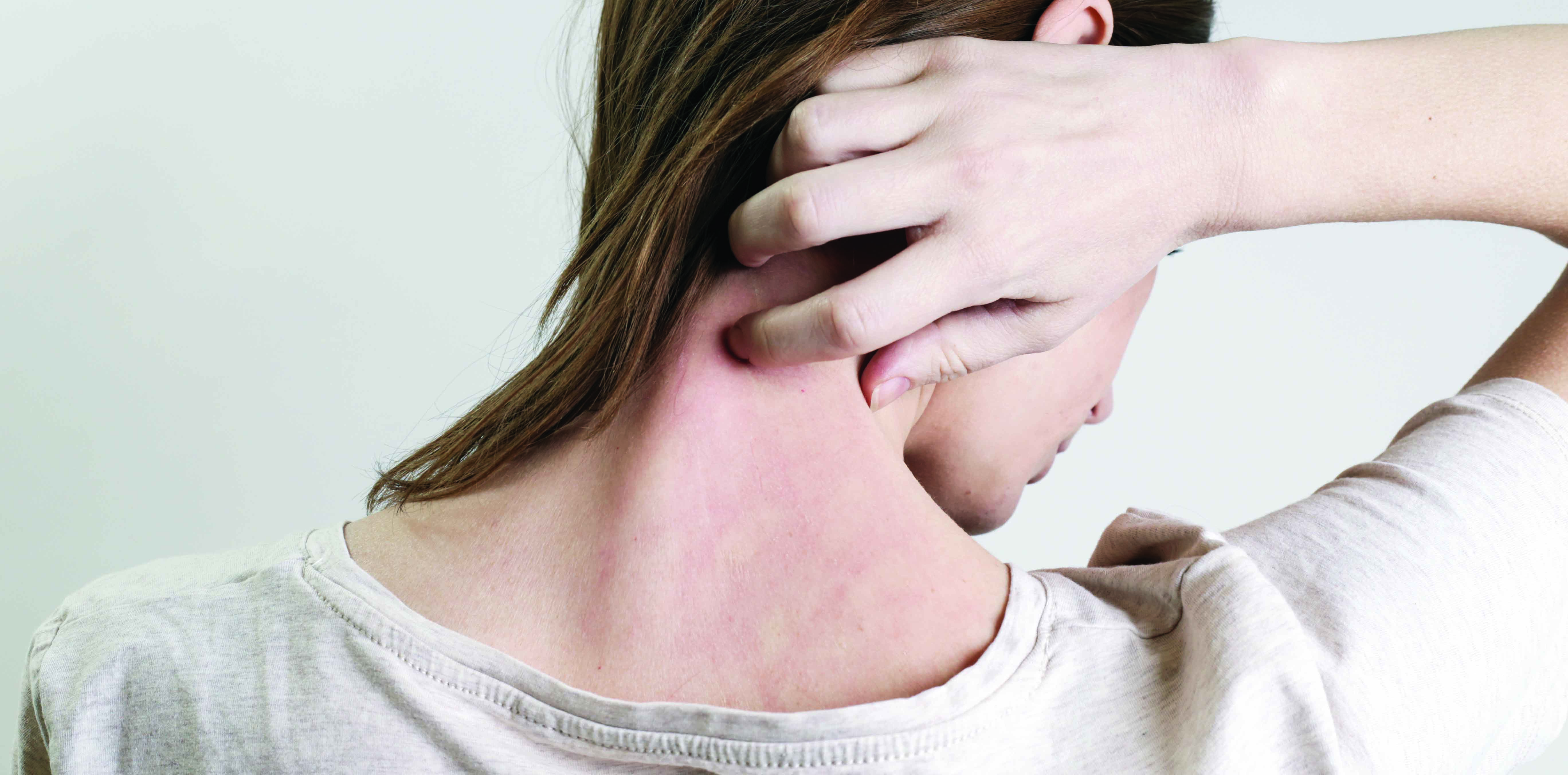
The effects of atopic dermatitis are more than skin deep, but biologic therapies are showing great promise
Atopic dermatitis, commonly known as eczema, affects up to 20% of children and 3% of adults worldwide and is a major public health burden.1-3
In the 2010 WHO article into the global burden of skin diseases, atopic dermatitis was ranked first by virtue of causing the most number of days that people were not at full health.2
The effects of the condition are more than skin deep. Children with poorly controlled atopic dermatitis suffer from reduced sleep and increased psychological problems.4 The feature of atopy (raised IgE) relates the disease to other allergic responses such as food allergies, allergic rhinitis and asthma.
This so-called “atopic march” describes the progressive acquisition of atopic diseases in a step-wise manner throughout childhood.5 Atopic dermatitis also has a substantial economic impact. A 2013 study in the United States showed that the direct cost for treatment might be as high as $3.8 billion a year.6
One of the most recent Australian studies from 2004 concluded that patients can incur substantial annual out of pocket costs for treatment products and medical consultations.7
DIAGNOSIS
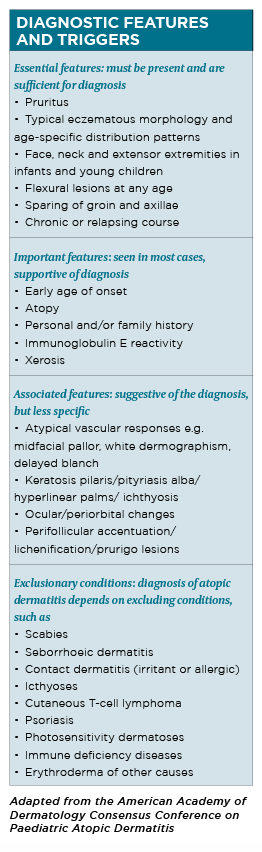
The diagnosis of atopic dermatitis is clinical, based on historical features, skin lesions morphology and distribution and associated clinical signs. Formal sets of diagnostic criteria have been developed by different groups.
The 1980 Hanifin and Rajka criteria are one of the earliest and most recognised sets of diagnostic criteria and require three of four major criteria and three of 23 minor criteria to be met.8
Although it is comprehensive and often used in clinical trials, the large number of criteria makes it difficult to use in clinical practice. Several international groups have since proposed modifications to improve the above criteria.
The United Kingdom working party, in particular, has simplified the Hanifin and Rajka criteria to a core set. 9 In 2003, the American Academy of Dermatology revised Hanifin and Rajka criteria that are deemed more streamlined and applicable to the full range of patient ages.10 (See table, page 28)
Validated scores to assess the severity of atopic dermatitis include the Eczema Area Scoring Index, Scoring Atopic Dermatitis and Patient-Oriented Eczema Measure.
PATHOGENESIS
Atopic dermatitis is immunologically characterised by the over-expression of T helper 2 (Th2) cytokines, including interleukin 4 (IL-4), IL-5 and IL-13 and chemokines (C-C motif chemokine ligand 17 (CCL17), CCL18 and CCL22, and IL-22, a Th22 cytokine.11
IL-4 and IL-13 are cytokines central to the pathogenesis of atopic dermatitis and produced mainly by Th2 cells.12, 13
IL-13 is thought to be a primary disease-inducing effector cytokine, while IL-4 works as a key amplifier of type 2 immunity by facilitating expansion of the CD4+ Th2-cell population in secondary lymphoid organs.14
IL-4 and IL-13 can activate and promote Th2 cells survival, induce differentiation and activation of myeloid and atopic dendritic cells, activation of B cells, stimulation of IgE class switching and eosinophil recruitment.15
Type 2 cytokines can cause:
1. Suppression of terminal differentiation proteins filaggrin, loricrin and involucrin.
2. Antimicrobial peptides inhibition.
3. S100As upregulation.
4. Epidermal hyperplasia induction.
5. Lipid synthesis suppression.
6. Spongiosis induction.11
IL-4 and IL-13 levels are found to be correlated with atopic dermatitis disease activity. 14
TREATMENT
General approach
As atopic dermatitis is a chronic relapsing disease, management includes patients/parents education, gentle skin care and regular use of emollients, as well as anti-inflammatory therapy.
Topical agents represent the mainstay of treatment. Severe disease may require phototherapy or systemic medications in conjunction with continued topical therapy. Factors that may exacerbate the condition should be identified and avoided.
Topical corticosteroids
Topical corticosteroids remain the first-line treatment. Their efficacy has been verified in more than 100 randomised controlled trials.16 Topical corticosteroids have anti-inflammatory, antiproliferative, immunosuppressive and vasoconstrictive actions, and have been demonstrated to decrease the acute and chronic inflammation of the disease and the associated pruritus.17
Topical calcineurin inhibitors
Topical calcineurin inhibitors include tacrolimus (0.03% and 0.1%) ointment for moderate to severe atopic dermatitis and pimecrolimus (1%) cream for mild to moderate conditions. The inhibitors suppress T-cell activitation and modulate secretion of cytokines and other proinflammatory mediators, and decrease mast cell and dendritic cell activity.18
Topical calcineurin inhibitors are useful for atopic dermatitis affecting the face and intertriginous areas where corticosteroid-induced skin atrophy may be of concern. They are also useful in managing frequent exacerbation of or persistent atopic dermatitis that would otherwise require continual topical corticosteroids usage.
Phototherapy
Phototherapy exerts its immunomodulatory effects via T-cell apoptosis induction, dendritic cells reduction and decreased expression of Th2 cytokines including IL-5, IL-13 and IL-31.19
Phototherapy including narrowband UVB and UVA have been demonstrated to improve atopic dermatitis and the associated pruritus. UVB treatment has also been demonstrated to reduce S. aureus colonisation of skin. Phototherapy is usually combined with topical corticosteroids.
The potential side effects of phototherapy include sunburn reaction and long-term treatment may be associated with photoaging and an increased risk of skin cancer.
Requirement to travel several times a week to a phototherapy centre may not be practical for some patients and it may be challenging for young children to cooperate with this treatment. However, its side-effect profile is still favourable compared with systemic immunosuppressive agents.
Traditional systemic anti-inflammatory therapy
Systemic anti-inflammatory medications may be administered for children and adults with moderate-to-severe disease who fail to respond adequately to optimised topical treatment. Combination of systemic treatment and topical corticosteroid therapy is frequently required to maximise therapeutic benefit.
Cyclosporin
Cyclosporin is a potent inhibitor of T-cell dependent immune responses and IL-2 production. Cyclosporin treatment leads to rapid improvement of atopic dermatitis in adults and children. However, due to potential side-effects, including nephrotoxicity and hypertension, it is used as a short-term treatment, working as a bridge between other therapies.
Azathioprine
Azathioprine is a purine synthesis inhibitor that reduces leukocyte proliferation. It is a treatment for moderate to severe atopic dermatitis in children and adults. Individuals with genetically determined low activity of the enzyme thiopurine methyltransferase (TPMT) have increased susceptibility to azathioprine-induced myelotoxicity.
By determining TPMT activity and/or genotyping for TPMT polymorphisms before starting treatment and adjusting dose accordingly, this can reduce the risk of treatment induced myelotoxicity. Azathioprine has a slow onset of action, with clinical improvement after one to two months and full benefit requiring two to three months of treatment.
Methotrexate
Methotrexate reduces allergen-specific T-cell activity. It is used in the treatment of refractory atopic dermatitis in adults and children together with folic acid supplementation. Maximum clinical effect is typically seen after two to three months of therapy.
Mycophenolate mofetil
Mycophenolate mofetil inhibits de novo pathway of purine synthesis, resulting in suppression of lymphocyte function. It is used in recalcitrant atopic dermatitis in adults and children, with maximum therapeutic benefit seen at two to three months of treatment.
Systemic corticosteroids
A short course of systemic corticosteroids may be considered for severe acute exacerbations of atopic dermatitis while phototherapy or immunomodulatory treatment is initiated.
Novel therapies
An increased understanding of the underlying immunopathogenesis of atopic dermatitis has led to development of new biologic therapies and small molecule drugs specifically targeting (inhibiting) immune and inflammatory mediators identified, including the Th2 cytokines IL-4 and IL-13, phosphodiesterase E4 and Janus kinases.
Dupilumab
Dupilumab is a fully human IgG4 monoclonal antibody administered subcutaneously. It targets the IL-4R subunit of the heterodimeric IL-4 and IL-13 receptors, resulting in blockage of the downstream signalling of IL-4 and -13, two important Th2 cytokines implicated in the immunopathogenesis of the condition.
Dupilumab is the first biologic therapy to have been approved for treating adult patients with moderate to severe diseases. Dupilumab treatment has been shown to alter the atopic dermatitis transcriptome in a dose-dependent fashion.
Differences in gene expression following administration of dupilumab include down-regulation of markers of epidermal proliferation, down-regulation of inflammatory mediators, upregulation of structural proteins, upregulation of lipid metabolism proteins, and upregulation of epidermal barrier proteins resulting in normalisation of skin.15
Dupilumab had been reported to significantly reduce serum levels of CCL17 (or thymus and activation-regulated chemokine), a key regulator of Th2-mediated immunity and a specific and objective biomarker of disease activity.20
In Australia, dupilumab was approved by the TGA in January last year for the treatment of moderate-to-severe atopic dermatitis in adult patients who are candidates for chronic systemic therapy. At the time of publication, dupilumab was not PBS listed.
In United States, the Food and Drug Administration approved dupilumab for use in adolescent patients with moderate-to-severe disease in March this year and there are studies under way investigating the efficacy and safety of dupilumab plus topical corticosteroids in patients six to 12 years old with severe atopic dermatitis, and the safety, pharmacokinetics and efficacy of dupilumab in children six months to six years old with severe disease.
Dupilumab comes as a single-dose 300mg pre-filled syringe for administration as a subcutaneous injection. The recommended dose is an initial dose of 600mg (two 300mg injections in different injection sites) followed by 300mg given every other week.
Phase III clinical trials in adults with moderate-to-severe disease have shown that dupilumab as monotherapy or in combination with topical corticosteroids (or topical calcineurin inhibitors if topical corticosteroids usage was inadvisable) improved multiple measures of disease severity, pruritus, sleep disturbance, anxiety and depression, and quality of life compared with placebo.21-23 Improvements in disease severity and itch with dupilumab treatment can be seen within first two weeks of treatment.
The most common side-effects of dupilumab either as monotherapy, or combined, include conjunctivitis, injection-site reactions and oral herpes. Other potential side-effects include skin infections and exacerbations of atopic dermatitis and nasopharyngitis.
The incidence of allergic conjunctivitis was two-fold higher in dupilumab plus topical corticosteroids recipients compared with placebo plus topical corticosteroids recipients in the CHRONOS study.22
Other adverse events occurring more frequently with dupilumab plus topical corticosteroids than with placebo plus topical corticosteroids in the above trial include eye pruritus, blepharitis and dry eye.
All cases of conjunctivitis were of mild or moderate severity and resolved with topical eye treatments.22
Patients should be advised to report new onset or worsening eye symptoms to their healthcare provider. Across all studies, the incidences of serious adverse events for dupilumab (with or without topical corticosteroids) leading to discontinuation were low. And dupilumab was not associated with any clinically significant laboratory abnormalities.21-23
Being a therapeutic protein, immunogenicity may potentially occur The 52-week CHRONOS study showed 2% of dupilumab plus topical corticosteroid recipients had anti-drug antibody responses, although production of such responses which did not seem to result in loss of efficacy. 22, 24
Hypersensitivity reactions (such as serum sickness, serum sickness-like reaction and generalised urticaria) occurred in less than 1% of dupilumab recipients.25
The use of live vaccines should be avoided in patients receiving dupilumab, but non-live vaccines may be administered concurrently.24,25 Therefore it is important to advise patients to discuss travel and vaccination plans prior to starting dupilumab so that they can be vaccinated appropriately in advance.
Other new biologic and small molecule therapies
Crisaborole ointment 2% is a phosphodiesterase inhibitor which gained TGA approval in February this year as topical treatment of mild to moderate atopic dermatitis in patients aged two years and older.
Cytokine-targeted therapeutic agents for atopic dermatitis in clinical development, including the IL-13 inhibitors tralokinumab and lebrikizumab, the IL-31 receptor A inhibitor nemolizumab; the IL-12/IL-23 inhibitor ustekinumab and IL-17 inhibitor secukinumab, which are already in clinical use for psoriasis treatment, are also being investigated for their possible roles in atopic dermatitis treatment.
The Janus kinase inhibitors, tofacitinib and baricitinib, are also in clinical development for atopic dermatitis treatment.
SUMMARY
Atopic dermatitis is an immune-mediated chronic relapsing inflammatory skin disorder characterised by a Th2 immune response and is a major public health burden.
Historically, there has been a lack of safe and effective long-term treatment options for patients with moderate-to-severe disease which does not respond adequately to first-line topical therapies and second-line treatment with phototherapy and systemic immunosuppressants.
Dupilumab is the first biologic agent approved for use in adults with moderate-to-severe disease. It binds to the IL-4R subunit of the heterodimeric IL-4 and IL-13 receptors, resulting in blockage of the downstream signalling of these two main cytokines implicated in the immunopathogenesis of the condition. Dupilumab can be used with or without topical corticosteroids or topical calcineurin inhibitors.
Improvements in disease severity and itch with dupilumab treatment can be seen within first two weeks of treatment. Across all studies, the incidences of serious treatment emergent adverse events for dupilumab leading to treatment discontinuation are low. The most common side-effects of dupilumab include conjunctivitis, injection-site reactions and oral herpes. Patients should be advised to report new onset or worsening eye symptoms to their healthcare provider. The use of live vaccines should be avoided in patients receiving dupilumab although non-live vaccines may be administered.
Dr Rose Mak is a consultant dermatologist in private practice (Epworth Freemasons Hospital East Melbourne and St John of God Hospital Berwick) and holds appointments at the Royal Children’s Hospital and the Skin and Cancer Foundation.
Acknowledgement: The author acknowledges Dr Richard Worrell for providing helpful discussions.
Conflict of interest: The author has no conflicts of interest to declare.
References:
1. Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006; 368: 733-743.
2.Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 2014; 134: 1527-1534.
3. Deckers IA, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidermatolgical studies. PLoS One 2012; 7: e39803.
4. Absolon CM, Cottrell D, Eldridge SM, Glover MT. Psychological disturbance in atopic eczema: the extent of the problem in school-aged children. Br J Dermatol 1997; 137: 241-245.
5. Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol 2009; 129: 1892-1908.
6. Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A. et al. Management of difficult-to-treat atopic dermatitis. J Allergy Clini Immunol Pract 2013; 1: 142-151.
7. Jenner N, Campbell J and Marks R. Morbidity and cost of atopic eczema in Australia. Australas J Dermatol 2004; 45(1): 16-22.
8. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1980; 92: 44-47.
9. Williams HC, Burney PGJ, Pembroke AC, Hay RJ. The UK working party’s diagnostic criteria for atopic dermatitis III: Independent hospital validation. 1994; 131: 406-416.
10. Eichenfield LF, Hanifin JM, Luger TA, Stevens SR and Pride HB. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol 2003; 49(6): 1088-1095.
11. Noda S, Kreuger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol 2014; 135(2): 324-335.
12. Brunner PM, Guttman-Yassky E, Leung DYM. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol 2017; 139(4, Supplement): S65-S76.
13. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017; 13(5): 425-437.
14. Leung D, Guttman-Yasky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014; 134: 769-779.
15. Hamilton JD, Suarez-Farinas M, Dhingra N, Cardinale I, Li X, Kostic A et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014; 134(6): 1293-1300.
16. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess 2000; 4: 1-191.
17. Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71: 116-132.
18. Krakowski AC, Eichenfield LF, Dohil MA. Management of atopic dermatitis in the pediatric population. Pediatrics 2008; 122: 812-824.
19. Gambichler T, Kreuter A, Tomi NS, Othlinghaus N, Altmeyer P, Skrygan M. Gene expression of cytokines in atopic eczema before and after ultraviolet A1 phototherapy. Br J Dermatol 2008; 158 (5): 1117-1120.
20. Kakinuma T, Nakamura K, Wakugawa M, Mitsui H, Tada Y, Saeki H et al. Thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001; 107(3): 535-541.
21. Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335-2348.
22. Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017; 389 (10086): 2287-2303.
23. De Bruin-Weller M, Thaci D, Smith CH, Reich K, Cork MF, Radin A, Zhang Q et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis and an inadequate response or intolerance to ciclosporin A r when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018; 178(5): 1083-1101.
24. EMA. Dupixent 300mg solution for injection in pre-filled syringe: EU summary of product characteristics. 2017. http://www.ema.europa.eu. Accessed 6 April 2019
25. US FDA. Dupixent® (dupilumab) injection, for subcutaneous use: US prescribing information. 2017. https://www.fda.gov. Accessed 6 April 2019