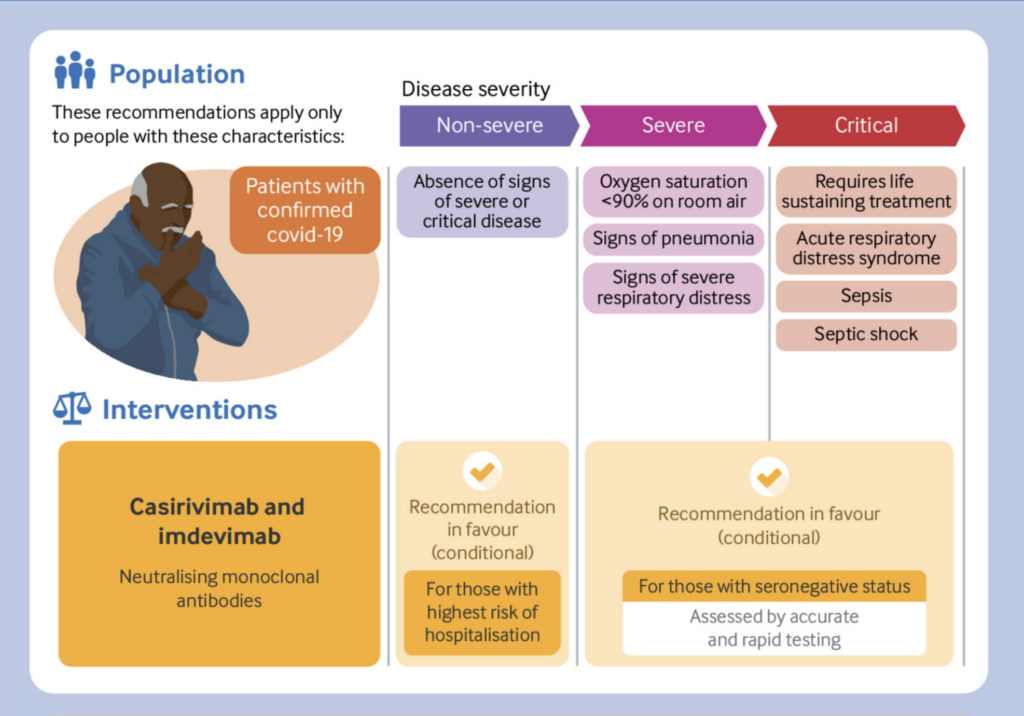
And the WHO has recommended the use of casirivimab-imdevimab in certain groups.
Welcome to The Medical Republic‘s Covid Catch-Up.
It’s the day’s covid-19 news in one convenient post. Bianca Nogrady is taking a well-earned fortnight off, so for now please email penny@medicalrepublic.com.au with any tips, comments or feedback.
- Victoria offers GPs grant payments to speed the vaccine rollout
- WHO recommends an antibody cocktail for non-severe cases
- TGA prepares to approve home tests
- Booster shots in the US
- The latest case numbers around the country
Victorian Premier Daniel Andrews will give grants of $10,000 and $4000 to GPs and pharmacies in 11 LGAs to give the vaccine rollout a little kickalong.
Announcing the “common sense” move in today’s press conference, he said the payments would allow GPs to “operate additional hours because they can employ some additional staff” or pharmacies to rent additional space.
Practices will be invited to express interest over the next week.
The WHO has updated its living guideline for treating covid patients, now conditionally recommending the monoclonal antibody combination of casirivimab-imdevimab for non-severe patients at highest risk of hospitalisation, and for severely ill patients who are seronegative.
The update is based on new preprints of four randomised studes in patients with non-severe illness and data from a subgroup of the large RECOVERY trial in the UK on patients with severe illness.
“Although there is no established decision tool to identify those at highest risk of hospitalisation,” the guideline says, “factors that substantially increase risk include no prior vaccination, older age, immunosuppression, and the presence of chronic conditions.”
It also concedes the problems of cost, inconvenience (the drugs are administered intravenously) and limited supply.
This sixth update comes on top of prior recommendations for the use of interleukin-6 receptor blockers and systemic corticosteroids for patients with severe or critical illness; and against the use of ivermectin, lopinavir-ritonavir and hydroxychloroquine in anyone regardless of severity.
Rapid antigen testing may soon be approved for use by individuals in the home. The TGA has published information for potential suppliers of rapid point-of-care covid testing, saying it was “progressing work that would allow the provision of self-tests (home-use tests) for covid-19 in the future”.
The US CDC director Rachelle Wolensky has overridden her agency’s own advisory panel and recommended a third Pfizer shot for people whose jobs put them at high risk of getting covid, in addition to people 65 and older, nursing home residents and people 50 to 64 with underlying medical conditions, as recommended by the CDC panel.
The panel also recommended that younger adults with underlying medical conditions may receive a booster if they choose.
Previously, an FDA advisory panel had contradicted the Biden administration by declining to recommend booster shots for the whole population, instead recommending them for the older and medically susceptible and those with high-risk jobs.
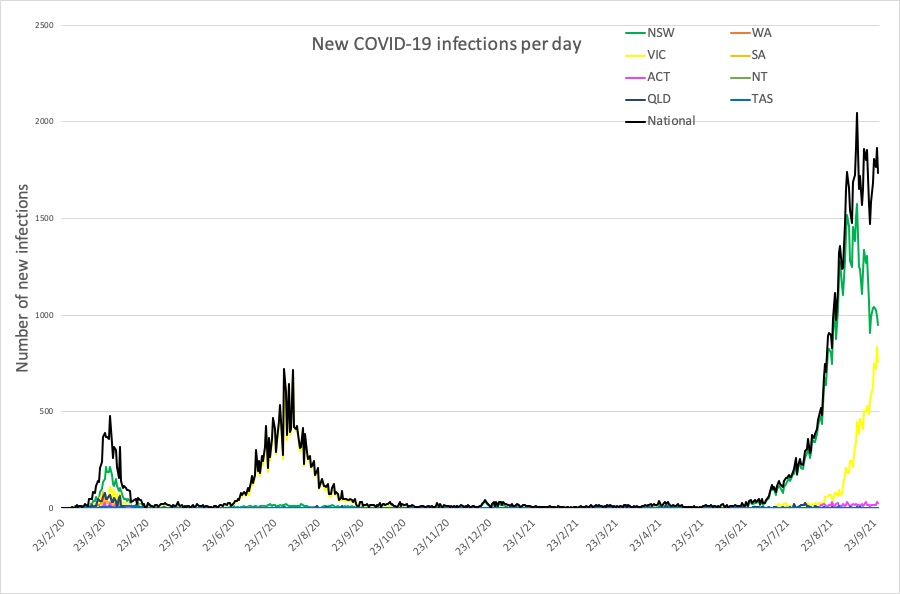
Here are the latest covid infection numbers from around Australia to 9pm Sunday:
National – 97,540 with 1231 deaths
ACT – 874 (25)
NSW – 58,931 (948)
NT – 209 (1)
QLD – 2022 (0)
SA – 901 (1)
TAS – 235 (0)
VIC – 33,273 (758)
WA – 1095 (0)