It’s a revolutionary technology, but how to use it has raised concerns within the scientific community
It’s a revolutionary technology, but how to use it has raised concerns within the scientific community
Back in December, in Washington DC, 500 experts from the world of science, ethics and medicine gathered to discuss one of the most exciting developments in medical science in decades. For three days, the International Summit on Human Gene Editing debated the emergence of new biochemical tools that, theoretically, could prevent the onset of some of humanity’s most debilitating conditions and illnesses.
The sense of anticipation at the summit, watched by 3000 people online in addition to those present in the US capital, was perhaps best encapsulated by biology professor Dr David Baltimore, president emeritus at the California Institute of Technology, and chairman of the summit organising committee.
“We could be on the cusp of a new era in human history,” Dr Baltimore declared in his opening address. “Today, we sense that we are close to being able to alter human heredity.”
Yet amid that immense sense of excitement and optimism, as real as it is, comes much angst.
Dr Baltimore followed up his grand opening statement with more sobering words.
“Now we must face the questions that arise,” he told delegates. “How, if at all, do we as a society want to use this capability?”
The capability in question is a gene editing technology called CRISPR, a memorable acronym at odds with its convoluted name: Clustered Regularly Interspaced Short Palindromic Repeats.
It’s a unique technology, said to be the greatest advance in the field since PCR. Put simply, CRISPR enables scientists to edit parts of the genome by cutting out, replacing or adding bits to the DNA sequence. It’s also been described as DNA scissors that only cut where you tell them to.
At its core – and what has the medical and scientific world so enthralled – is that it can be programmed to identify and correct malfunctioning genes with unprecedented ease and precision.
Its uses in research are probably countless, and its potential to genetically alter an organism – plant or animal – or even a species, has been said to be both tremendously exciting and chilling at the same time. For example, already scientists have used the technology to create monkeys with targeted mutations and created resistance to HIV in human white cells in the lab.
While gene editing techniques have been the subject of laboratory exploration for many years, the relatively simple and precise nature of CRISPR has accelerated the possibility that in the near future – maybe within five or 10 years – we could alleviate the suffering of countless people by correcting mutations responsible for illness and disease. Imagine if we could edit out the gene for cystic fibrosis, for example. Or muscular dystrophy.
But amid the eagerness to realise the potential of CRISPR, many profound questions are being raised over the ethical, moral and social issues associated with some uses of the technology. The controversy predominantly surrounds the ability for CRISPR to alter the genome of embryos, alterations that would not only affect that one individual, but be passed on to subsequent generations. While the ability to stop hereditary disease in its tracks sounds extraordinarily attractive, debate has raged over the unknown long-term health issues such procedures may inflict on future generations, and the wider impact it could have on society.
The ethical and moral arguments are plentiful, not least surrounding the possibility, and a plausible one at that, of designer babies, with parents-to-be effectively able to hand-pick physical characteristics of their child by tinkering with their DNA. While that is likely to be some way off, the prospect is no less real for that.
At what point does society’s desire to eradicate life-shortening illnesses – clearly the holy grail of CRISPR – begin to fuse into more cosmetic alterations and enhancements? Writing in The Wall Street Journal, Marcy Darnovsky, executive director for the US Centre for Genetics and Society, warned of the creation of “genetic haves and have-nots”. “It’s all too easy to imagine fertility clinics offering offspring upgrades to affluent parents, and competitive pressures to make sure your children aren’t left behind,” Dr Darnovsky wrote. “Not far down that road we could see the emergence of genetic haves and have-nots, with new forms of inequality and discrimination.”
In an indication of the seriousness of the discussion at hand, December’s Washington summit recalled historical human rights abuses and the practice of eugenics in the early 20th century, a hugely contentious movement that sought to improve the genetic quality of the human population.
“Only when the Nazis took eugenic ideas to horrific extremes was the concept thoroughly discredited,” the summary noted.
But Matthew Beard, a moral philosopher at The Ethics Centre in Sydney, warned against depicting CRISPR, and technologies like it, within the confines of a slippery slope and worst-case scenarios. “The first thing we need to ask ourselves is what are the reasons for tinkering with DNA?” he said. “And for a lot of reasons it’s to do with genuine and morally praiseworthy goals.
“We are trying to reduce suffering and to combat illness and disease, we’re trying to improve capacities that deserve or need to be improved. So intrinsically the goals we are trying to achieve are good ones.
“The interesting thing about the phrase slippery slope is that it’s a logical fallacy. When we do many things… our actions usually have a range of consequences, some of which are desirable and some undesirable. The fact there are possible undesirable consequences isn’t sufficient to say that action should not be taken or is unethical,” Beard said.
“We need principles in place to try to manage the potential negative consequences, but merely pointing to the possibility of negative outcomes is not a sufficient ethical reason not to pursue a piece of technology.”
Beard also rejected the oft-cited objection that such intervention in the human genome is tantamount to playing God. He described such thinking as dated, arguing it belonged in an era within a moral context that was no longer relevant.
“A lot of the arguments around avoiding any forms of genetic editing are kind of dated or based on a kind of naturalism or quasi-religious morality about not wanting to play God,” he said. “And look, those arguments are worth having, but the problem with assertions like this is that they’re made without basis, or without showing why it would be bad for us to play God.
“In the West we effectively live in a secular society and increasingly our ethical framework is non-religious. The backdrop that formed those arguments (about playing God) has disappeared. The context in which those arguments were made is no longer there, so we need to re-evaluate them to see how they stand up in the current context.”
Furthermore, a moratorium on any type of research around genetic editing is “really quite conservative”, Beard said, “and arguably too conservative, given all of the ethical benefits that are offered by this technology”.
Loane Skene, Professor of Law at Melbourne University and a member of the NHMRC Legislation Review Committee on Human Cloning and Embryo Research, said she too was not attracted by the playing God argument, “partly because we do it all the time”.
“People have vaccinations for example and we could say that is playing God,” she said. “You could also argue that God has given us the power to make decisions. All the time we are making decisions that we hope will improve our welfare and the welfare of people around us, so I am not persuaded by the playing God argument.”
Skene also suggested the debate surrounding the creation of designer babies was a red herring, arguing the invasive nature of embryonic editing would deter any unethical march towards designer babies. In any case, “you wouldn’t find a doctor who would be prepared to do that”, she told The Medical Republic. “What we should be focusing on is the safety aspects both to the child to be born and to the children they might have,” Skene said, explaining that while there will always be risks with any medical discovery, actions that could have far-reaching implications for future generation takes risk to another level.
“There has always got to be that first person… but we need to proceed with caution,” she said. To progress CRISPR further, and for scientists to continue on their journey of discovery, legislative change will be needed. Most notably, and controversially, will be the need to conduct research on embryos.
Earlier this year, the UK Human Fertilisation and Embryology Authority granted permission for scientists in Britain to edit the genomes of human embryos for 14 days, the world’s first endorsement of such research by a national regulatory body. Critically, however, the permission stops short of allowing scientists to implant them into women.
“CRISPR research in very early stage human embryos may be permissible in most Australian states and territories [but] to do this, researchers would first need to gain a specific licence from our national regulator, the National Health and Medical Research Council,” Dr Ainsley Newson, associate professor of Bioethics at the University of Sydney, said.
“However, using CRISPR to edit the genome of an embryo to then implant it in a woman’s uterus would be illegal. At the moment, the law seems to reflect ethical consensus. But if we do reach a point where we agree it’s ok to use CRISPR to change an embryo to prevent a debilitating disease, current laws would have to change,” Newson said.
Dr Newson noted that current regulations in Australia already prevented CRISPR being used to “make a baby-to-order” but “it will also stop us from helping families living with debilitating genetic conditions”.
“This is not to say we should do this right now, but in the rush to prevent designer babies let’s not forget some of the more medically justified uses for CRISPR. The key will be to appropriately balance these more justifiable uses with its less desirable effects.”
Professor Martin Delatycki, director of the Bruce Lefroy Centre of Genetic Health Research at the Murdoch Children’s Research Institute in Melbourne, predicted CRISPR could be used for patients with existing conditions within 10 years, but suggested the modification of DNA in embryos was a lot further off.
“There is appropriate concern about the impact of altering an embryo in ways where we are not 100% sure what will happen, and the multi-generational impact that may have,” he said. “I think it will happen but certainly in countries with very sophisticated regulatory processes, it’s a long way off.
“But I certainly don’t buy into the argument that we shouldn’t be doing it because someone might use it for cosmetic reasons. You can put regulations in place, and also, given the cost and effort there would be very few people who would want to do that. It would be a pity not to treat severe genetic disease because of concerns it will be misused. That is up to society to put safeguards in place to stop that happening.”
In addition to grappling with the ethical dilemma, concerns have emerged over unintended consequences of CRISPR and the potential wider impact on society. One fear is that people with genetic abnormalities could be further marginalised and devalued if their communities dwindle in number.
Leticia Keighley, whose four-year-old son Wade has Down syndrome, said society would lose intrinsic value if attempts were made to eradicate such conditions. She warned it could lead to further discrimination and ignorance about such communities as society began to expect parents-to-be to bear only healthy children.
“As a parent I find it very distressing because I look at my son and see him as being incredibly valuable,” she explained. “Yes, he is different, but he has value and intrinsic qualities. I don’t see Down syndrome as an illness or disease that can be plucked out of that person and they will still be who they are. I see it as being interwoven with their character.
“With something like cystic fibrosis, it feels to me that if the person didn’t have it, they would be the same person, just healthier. With Down syndrome, I see it as an integral part of who that person is. And it is part of human diversity.”
Keighley added that society already regarded people who were different as a “problem that needs fixing”.
“We don’t accept difference very well and there are many social barriers in place for people who have a disability,” Keighley said. “Of course Wade will have his own hurdles to overcome, but I believe he has more hurdles because of society’s views on Down syndrome.”
Asked if those barriers would get higher if the number of people with Down syndrome falls, Keighley said: “Absolutely. There is already an assumption that people with Down syndrome have nothing to contribute. And if you have less people with Down syndrome you will have less money for interventions and therapies, support and services.”
However, while sympathetic to Keighley’s view, Professor Delatycki said these fears should not stand in the way of medical progress that could ease much suffering.
“It is something we have to be very conscious of as a society,” he said. “There will always be disability and things we can’t prevent or treat and those individuals and families must be looked after and respected. But it’s not a good enough reason to say we shouldn’t proceed with technology that could allow people a better quality of life.”
Whatever the future holds for CRISPR, and technologies like it, and the impact that could be felt on individuals, families, advocacy groups and society at large, what is clear is the need for rational and lengthy debate involving all stakeholders over the varied and complex issues it involves.
In the words of Dr Newson: “In reflecting on its development and possible use we should be wary of always jumping on the worst possible conclusions and using these to argue against the technology as a whole. Instead, let’s proceed with open and transparent dialogue involving the public, lawmakers and researchers.”
What is CRISPR?
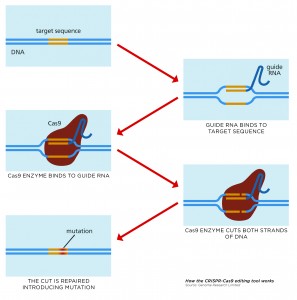
CRISPR is a game-changing gene-editing technique, its initials standing for Clustered Regularly Interspaced Short Palindromic Repeats.
Although called CRISPR for short, the system is more correctly known as CRISPR-Cas9 because it consists of two key molecules that together can introduce a change at a specific location in the genome.
These are:
- Cas9, an enzyme, or nuclease, which acts as molecular scissors to snip DNA so that bits can be added or removed.
- A piece of RNA called guide RNA (gRNA), which has RNA bases complementary to those of the target DNA sequence in the genome. This gRNA is designed to find and bind to the desired sequence of DNA, ensuring the Cas9 enzyme snips the right place.
Once the cell is transfected with the CRISPR system, and the DNA cut at the desired location, the cell recognises that the DNA is damaged and tries to repair it. But the DNA repair machinery is not 100% accurate, and a few bases are often lost around the site of the cut when it is repaired.
This loss of bases represents a permanent change, or mutation, in the genome and will affect the activity of the gene in which it is located. This may mean the gene doesn’t function properly or doesn’t function at all. Scientists can therefore use CRISPR-Cas9 to target and mutate one or more genes in the genome of a cell.
According to one its inventors, Jennifer Doudna, a biochemist at the University of California, her team stumbled across the technology during a project aimed at discovering how bacteria fight viral infections.
“Bacteria have in their cells an adaptive immune system called CRISPR that allows them to detect viral DNA and destroy it,” Doudna said.
“Part of the CRISPR system is a protein called Cas9 that is able to seek out and cut, and eventually degrade, viral DNA in a specific way. It was through our research to understand the activity of this protein Cas9 that we realised we could harness its function as a genetic engineering technology, a way for scientists to delete or insert specific bits of DNA into cells with incredible precision.”