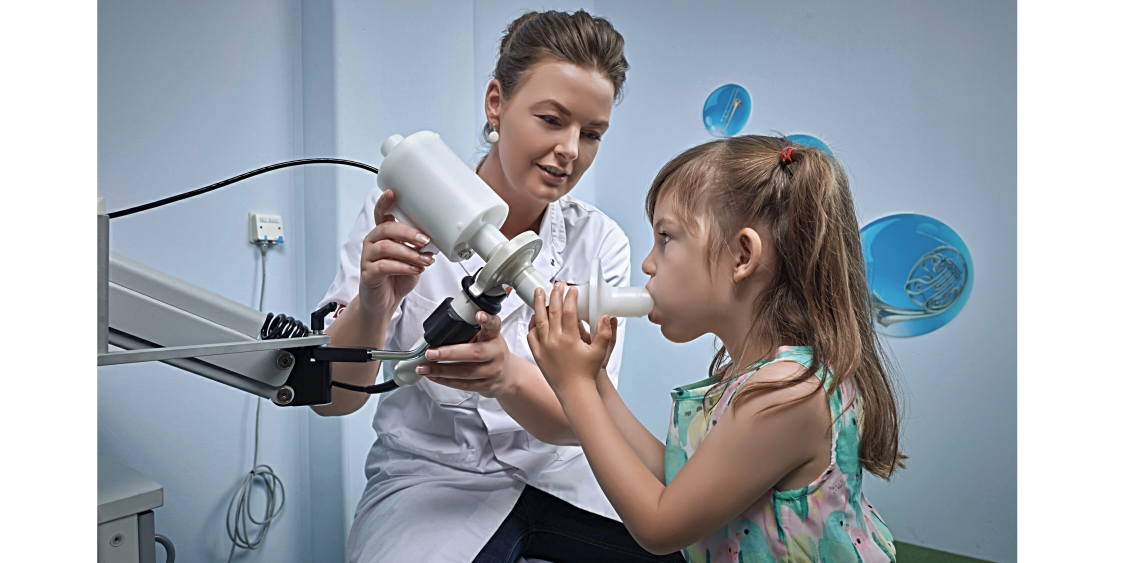
An electronic "nose" developed in The Netherlands is able to sniff out subtle differences in the chemical composition of the breath of patients with lung diseases
Professor Anke-Hilse Maitland-Van Der Zee used to be a genetics researcher, but it was finicky, often frustrating work.
“Sometimes we found associations but usually they were not very strong,” she says. “It was always very hard to replicate them.”
Now, she runs a lab at Amsterdam UMC that uses electronic noses to detect signs of asthma, COPD and lung cancer in the breath of patients. It sounds fantastically futuristic, I know. But, as she puts it, “the results we see with exhaled breath are actually far stronger than what I’ve ever seen in genetics”.
An electronic nose is quite similar to a human nose. It senses the volatile organic chemicals that we humans would describe as “smells”.
While human noses have around 400 types of scent receptors, the “SpiroNose” used by this Dutch research team has just seven receptors.
But that’s enough for the eNose to sniff out subtle differences in the chemical composition of the breath of patients with lung diseases.
The procedure is quick and simple; patients just have to rinse their mouth, breath normally five times and then inhale completely and exhale into the eNose. As long as the patient hasn’t been drinking alcohol, the eNose should get a good reading and send the data to the cloud.
The Amsterdam UMC’s BreathCloud project has now collected breath samples from around 3,000 people who had a diagnosis of either asthma, COPD, lung cancer or who were healthy.
eNoses purposely don’t identify each chemical in the breath individually like methods based on mass spectrometry do.
Rather, they capture “breathprints” – unique patterns that can be used to sort patients into different clinical categories.
“Breath is a good thing to measure for respiratory disease because it’s coming directly from the compartment that we are interested in, namely the lungs,” says Professor Maitland-Van Der Zee.
Human breath contains lots of uninteresting chemicals such as nitrogen, oxygen, carbon dioxide and water.
But it also contains traces of thousands of volatile organic compounds (VOCs), which together “tell a story about what’s going on in the lungs”, says Professor Maitland-Van Der Zee.
VOCs, for example, can tell researchers whether an asthmatic patient has eosinophilic or neutrophilic inflammation in the lungs.
VOCs can also tell you which microorganisms have colonised the lungs. Each bacterium produces its own airborne chemicals, potentially allowing researchers to diagnose infections such as tuberculosis.
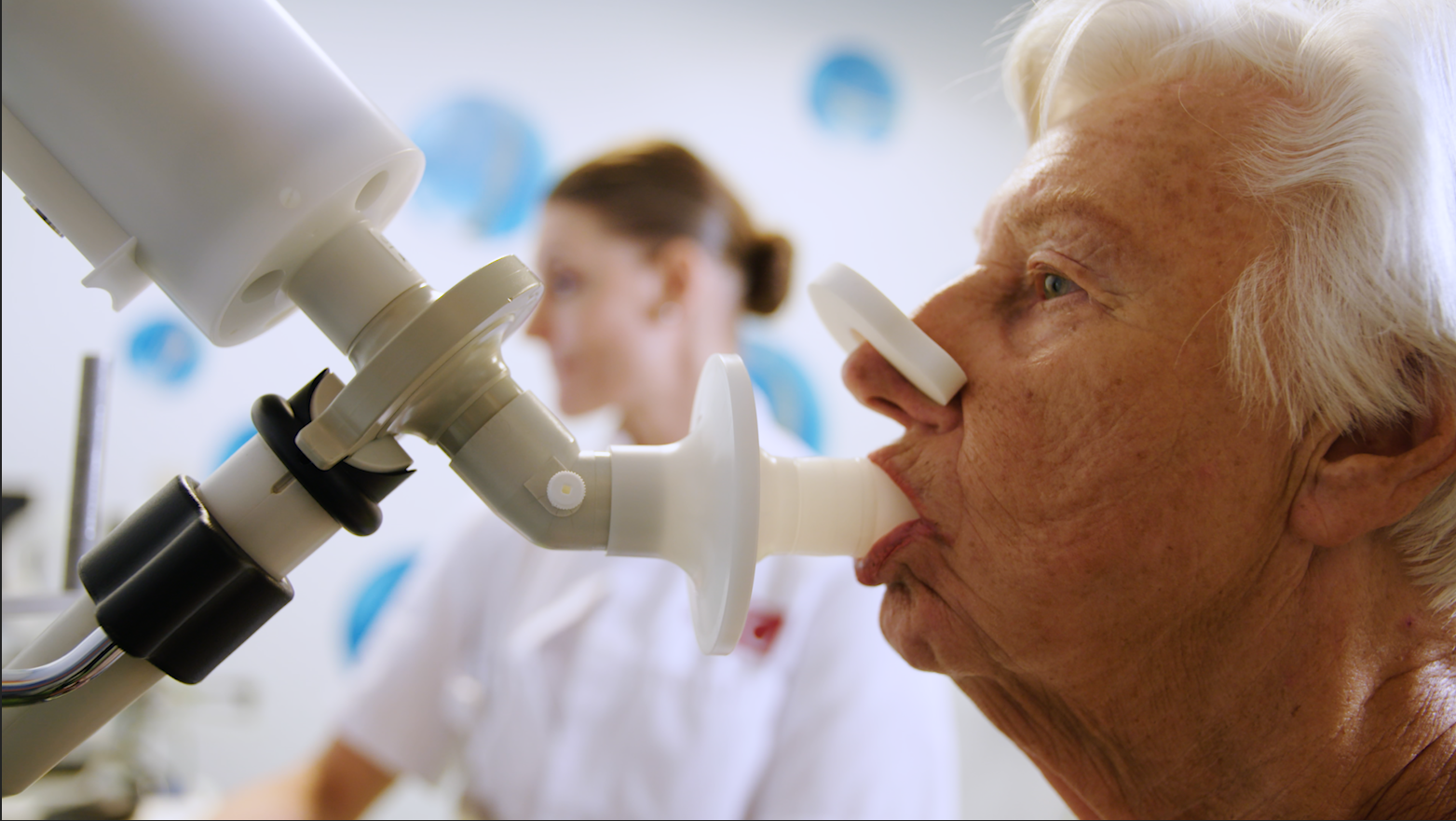
The lungs are in contact with many blood vessels, so the VOC signature of the bloodstream is also present in the breath.
“There are a lot of things you can measure in exhaled breath,” says Professor Maitland-Van Der Zee.
Research on exhaled breath has been going on for decades, so the fundamental usefulness of the approach has been quite well-established.
A review of 13 studies, published in Pediatric Pulmonology in 2017 and co-authored by Professor Maitland-Van Der Zee, reported that breathomics could predict asthma in children with 80 to100% accuracy using models including 6-28 VOCs.
The review concluded that breathomics could diagnose paediatric asthma just as well, if not better, than current techniques such as spirometry, FeNO and sputum analysis.
“However, standardisation of procedures, longitudinal studies, as well as external validation are needed in order to further develop breathomics into clinical tools,” the authors wrote.
A study published in the European Respiratory Journal in 2018 found that the eNose could accurately classified patients by asthma and COPD phenotypes that had previously been defined in the literature.
The eNose separated patients with asthma and COPD into five clusters based on a principal-component analysis.
Interestingly, each cluster contained a mix of patients with COPD and asthma, which reflected the known overlap between these conditions.
The clusters had significantly different eosinophil and neutrophil blood counts. This indicated that the eNose could be a useful tool for discovering the type of inflammation driving the disease and, may be useful for selecting the best drug for the patient.
“Another area where the SpiroNose can be very successful is in lung cancer,” says Professor Maitland-Van Der Zee.
In an abstract presented at American Thoracic Society conference in 2019 (which has not yet been peer reviewed or published), the Dutch researchers showed that the eNose could distinguish between patients with COPD who did and did not develop lung cancer after one year.
“Patients with COPD are at high risk of developing lung cancer, but no biomarkers have been reported to identify these patients,” the authors wrote.
“These results show that eNose assessment may detect early stages of lung cancer and may therefore be of value in the screening of patients with COPD.”
Another paper that has been submitted for publication by the Dutch team showed that the eNose could predict whether patients with advanced lung cancer would respond to last-resort immunotherapy treatments (nivolumab and pemrolizumab) with greater accuracy than the current test (the PDL1 test).
“The other area where the eNose might work in the future is recognising infections,” says Professor Maitland-Van Der Zee.
For example, patients with cystic fibrosis are often on long-term antibiotics to supress lung infections, which kills the healthy bacteria in their gut. The eNose might be able to monitor the infection in the lungs, help doctors select targeted drugs and reduce the length of antibiotic regimens.
The SpiroNose technology has now been commercialised by a former PhD student of the lab.
The cost of running the technology is cheap (just the price of a new mouthpiece each time), but it is expensive overall if you don’t use the machine a lot, says Professor Maitland-Van Der Zee.
Professor Maitland-Van Der Zee doesn’t have any financial ties to Breathomix, the company building the SpiroNose, but she has received some funding from GlaxoSmithKline, AstraZeneca and Boehringer Ingelheim in the past (this funding was not tied to her eNose research, however).
But what do clinicians make of breathomics? Will this technology ever make it into practice?
“I think it has promise but it’s certainly not ready for prime time yet,” says Professor Guy Marks, a respiratory physician and the lead researcher at the Woolcock Institute of Medical Research in Sydney.
“This is just another example of the -omics type approach: genomics, proteomics, metabolomics and now they are calling this breathomics,” he says.
Traditionally, diagnostic tests have exploited knowledge about disease mechanisms.
This new field of “-omics” research is less fussed about mechanisms and is more interested in predicting an outcome based on pattern recognition, says Professor Marks.
“And, in this case, it’s saying, ‘Exhaled breath tells us about the airways and the lungs and is it possible to deduce information by analysis of the fingerprint or the characteristics of the exhaled breath that can be informative for clinical purposes’.
“And it looks like, on the basis of quite a lot of early work, that the answer is: yes, it can be informative for the diagnosis of a whole lot of diseases.
“But it needs to go beyond what has been shown so far. Ultimately, to be useful in clinical practice, it needs to be shown that decisions that are based on the outcome of this test are safe and effective in improving patient outcomes.
“And then it needs to be shown that it can be implemented feasibly in a variety of clinical practice settings and that in implementing it in that setting is more beneficial and cost-beneficial than alternatives.
“So, just saying that it predicts some other test result is one step along the way to developing these tools, but it is not the end of the process.”