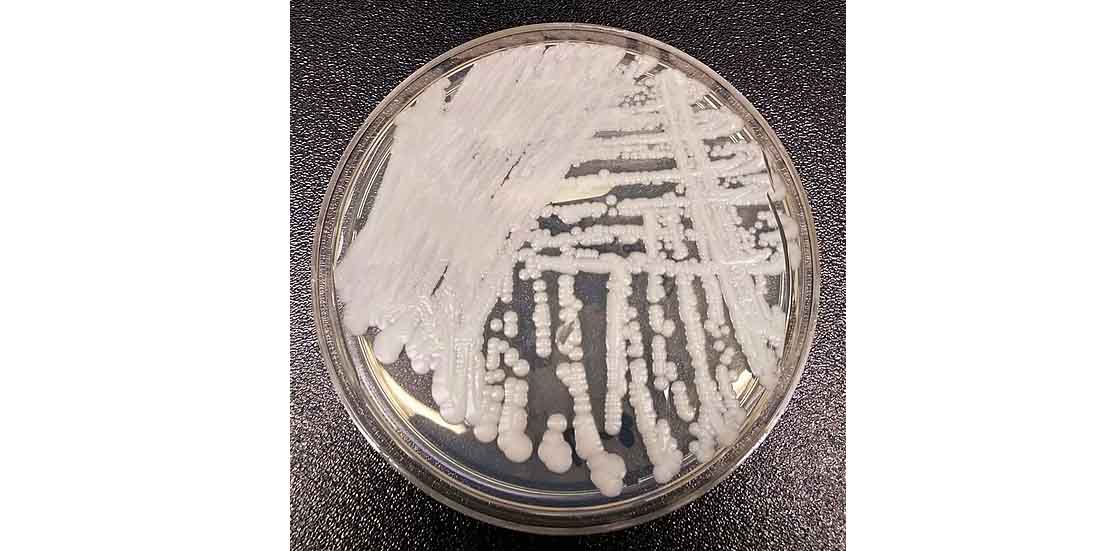
Candida auris is a fungus which breeds most commonly in healthcare settings. It's cause for concern because it's hard to detect, and is resistant to many anti-fungal drugs
We’ve recently heard a lot about Candida auris, a deadly, multidrug-resistant fungus emerging around the world. This pathogen has been labelled the new “fungal superbug” and poses a significant threat to public health.
C. auris tends to infect people with weakened or compromised immune systems. It thrives on the skin where it persists for long periods.
It also sheds into the patient’s environment – commonly a hospital or aged care home – and sticks to and survive on surfaces very well.
In hospitals, invasive fungal infections, particularly candidiasis caused by C. auris, can jeopardise patient safety and worsen outcomes after cancer treatment or surgery.
But it’s tricky to diagnose, and, importantly, C. auris has a resistance profile that can make it very difficult to treat.
Many people who get sick with an infection caused by C. auris won’t survive.
Read more: A frenemy fungus provides clues about a new deadly one
How common is this deadly fungus?
C. auris was first identified in 2009 in Japan.
Infections have since been reported in a range of countries, but the prevalence of C. auris has likely been underestimated in most places. This is because it’s hard to identify, and surveillance programs, where they exist, may not capture all cases.
The Centres for Disease Control and Prevention in the United States report that 617 cases of C. auris have been detected in the US.
Australia has had very few cases, and the cases we have seen have mainly been acquired overseas.
The Victorian health department notes hospital stays in a number of affected areas such as the UK, India, Pakistan, China, South Africa and parts of the US may pose a risk.
How does it manifest and how is it diagnosed?
C. auris seems to most commonly colonise and infect people who are already sick or have compromised immune systems, such as cancer or transplant patients, or hospitalised patients who are very young or very old.
Risk factors include suppression of the immune system (such as via medication after organ transplants to prevent organ rejection), recent surgery, diabetes, and the presence of an in-dwelling medical device such as a catheter.
Read more: Deadly frog fungus has wiped out 90 species and threatens hundreds more
The presence of the fungus on the body, called colonisation, doesn’t necessarily cause illness.
But invasive candidiasis (that is, invasive infection with Candida species) can infect the bloodstream (candidemia), central nervous system and internal organs.
When the infection spreads to the bloodstream, it can manifest as sepsis, with symptoms including fever, rapid breathing, muscle pain, and confusion.
Just like serious bacterial infections, C. auris may form abscesses in different parts of the body and may require surgery.
It can be difficult to diagnose fungal infections and accurately identify the species that has caused the infection. C. auris is very similar to other common fungi of the Candida genus and can be misidentified.
In most Australian hospitals, we use a blood culture diagnostic test. This is time-consuming and relies on specialists to accurately identify the pathogen.
Alternatives such as molecular diagnostic tests are not routinely used in many hospitals due to their cost, and, even when they are, they may not be able to accurately identify the type of fungus that has caused the infection.
However, these tests are improving and will become more widely available soon.
Outcomes for people who contract C. auris
Patients with weakened immune systems who have candidemia or invasive candidiasis have a 30-60% chance of dying after becoming infected.
But it can be very difficult to tell if these patients die from their infection, or die with their infection, given infection typically occurs when a person is already very sick.
Read more: Explainer: why do we get fungal nail infections and how can we treat them?
Another problem is that treatment with antifungal drugs is routinely delayed. The time between the taking of a blood sample and the delivery of a test result often extends beyond 48 hours, which can lead to delayed or inappropriate treatment (because the cause of infection is still unknown).
Delaying antifungal therapy is associated with an increased risk of death.
For these reasons, we need better tests to diagnose fungal infections.
Why resistance has developed
C. auris has developed resistance to several different antifungal drugs.
While its resistance profile varies geographically, C. auris is almost universally resistant to fluconazole, a dependable drug in the azole class of antifungal drugs – one of the four main classes of existing antifungal drugs.
We need to learn more about this, but it’s been suggested the prevalence of antifungal use in the environment contributes to the acceleration of resistance.
For example, in the presence of azole-based pesticides, we’ve seen the emergence of azole-resistant Aspergillus, another genus of fungi. Resistant strains then reproduce in the soil, and infections may be contracted from spores in the air that are inhaled.
Similar processes may also have led to the emergence of resistant C. auris, but we don’t know this for sure.
Read more: Five of the scariest antibiotic-resistant bacteria in the past five years
How can we control it?
C. auris is notoriously hard to clear. It poses enormous challenges for hospital cleaning and infection control.
In addition to disinfectants with antifungal activity, hydrogen peroxide vapour or UV light are now also used, when feasible, to clean contaminated environments.
Australia is on high alert, and hospitals screen patients who may have been exposed.
But we need continued vigilance, co-ordinated efforts to stop its spread in hospitals, and to diagnose it early. We also need to use antifungal agents wisely in human health and the environment.
Authors
Mis Head, Department Infectious Diseases, Peter MacCallum Cancer Centre, Peter MacCallum Cancer Centre
Ais Project officer, Department of Medicine and Radiology, University of Melbourn
Disclosures
Monica Slavin receives funding from National Health and Medical Research Council and Melbourne Health foundation and has received funding from Victorian State Government Fight Cancer foundation and Cancer Council. She has also received research and educational grants from Merck, Gilled and Pfizer
Karin Thursky receives funding from NHMRC
Arjun Rajkhowa and Megan Crane do not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and have disclosed no relevant affiliations beyond their academic appointment.
This article is republished from The Conversation under a creative commons licence. Read the original article.