Australian researchers are developing nanoparticles that might one day detect and kill cancer simultaneously
Imagine if you could shrink a machine down to the size of a protein and program it to find and destroy tumour cells.
This once sounded far-fetched, but for scientists working in the field of nanomedicine, it is now a realisable research goal.
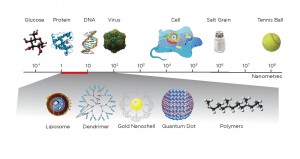
Nanoparticles – tiny structures between one and 1000 nanometers – can already be used to diagnose or treat some cancers.
So creating an all-in-one nanoparticle that can find a tumour, show up in an MRI or PET scan and then release drugs to kill the cancer cells – all at the same time – is the next logical step. This unification of therapy and diagnostics, known as theranostics, is highly feasible in the near future, say experts.
“I envision in the next 10 years we will be in a situation where a clinician will have a nanoparticle which will enhance imaging… but the same nanoparticle might have anti-cancer properties,” Dr Phoebe Phillips, head of the pancreatic cancer translational research group at UNSW, told The Medical Republic.
How do they work?
Nanoparticles are in the same size range of biological structures as DNA, proteins and viruses. They fit into a “Goldilocks zone” of particles that are much smaller than a human cell but larger than an individual molecule. By reducing medicine to the nanoscale, scientists can interact with biological systems in very targeted ways that have not been possible in the past.
The beauty of nanoparticles in cancer is that they can transport a variety of therapies specifically to tumour cells – drugs, gene therapies or imaging agents. Nanoparticle carriers are better than traditional therapies alone because they actively target tumour cells.
This is achieved by tethering specific disease-seeking antibodies or peptides on the nanoparticle surface. These chemicals latch on to glycoproteins that are overexpressed on the surface of cancer cells.
Then, nanoparticles can enter the cancer cell and release their payload.
This ability to target specific tissues or cells is tremendously beneficial, particularly for children who sometimes suffer life-long health problems as the result of aggressive treatment.
“Nanotechnology has the potential to reduce the off-target effects of the drugs and the toxicity,” says Dr Phillips.
Current uses
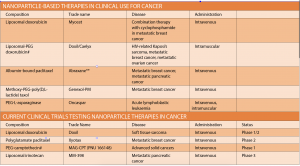
Nanotechnology is far from the stuff of science fiction; systems such of these are already in use. Doxorubicin liposomal (Doxil/Caelyx) became the first nanomedicine to be approved by the FDA in 1995 and was later approved by the TGA in 2008. Doxil is the drug doxorubicin encapsulated in a closed lipid sphere, or liposome, and is used to treat AIDS-related Kaposi’s sarcoma, breast cancer, ovarian cancer, and other solid tumours.
“Often when I speak to clinicians they are not even aware that what they are using is a nanoparticle application,” says Dr Phillips.
Another nanoparticle already used in cancer treatment is Abraxane. These 130-nanometre particles comprise the chemotherapy drug paclitaxel bound to albumin.
Because paclitaxel is not very soluble, it previously needed caustic detergents to make it bio-available. “So it was never used,” says Dr Phillips. With a nanoparticle carrier, however, the drug is readily absorbed, mitigating toxic effects.
Abraxane is now the first line treatment in pancreatic cancer. “It’s the only drug. It’s the best that we’ve got and it increases survival by only 16 weeks but that’s better than nothing,” says Dr Phillips. It is also used to treat breast and lung cancer.
Other nanoparticle-based therapies currently used to treat cancer include liposomal doxorubicin (Myocet) and methoxy-PEG-poly (D,L-lactide) taxol (Genexol-PM) for metastatic breast cancer; and PEG-L-asparaginase (Oncaspar) for acute lymphoblastic leukaemia.
What are they made of?
Of course, many different kinds of materials can be reduced to a nanosize, which means scientists have the ingredients to create a vast multitude of tailored imaging agents and drug carriers. As physicist Professor Dayong Jin, from the University of Technology Sydney says, “The solutions meet the problems”.
“You tend to break nanoparticles down into soft and hard,” says Professor Thomas Davis, nanotechnologist and director of the Australian Research Council Centre of Excellence in Convergent Bio-Nano Science and Technology (CBNS). “And the soft ones are really based around polymers or lipids and the hard nanoparticles are based around metallic nanoparticles or silica.”
Some of the members of the diverse nanoparticle family include:
- dendrimers (a synthetic polymer with a branching, tree-like structure);
- carbon nanotubes, metallic nanoparticles (iron oxide, gold);
- nanocrystals;
- quantum dots (made of semiconductor material such as silicon, cadmium selenide, cadmium sulfide, or indium arsenide);
- lipid-based amphiphiles (liposomes, hexasomes, cubosomes).
External diagnostics
The high costs and red tape involved in running clinical trials slows the creation of nanoparticle diagnostics for cancer that operate in vivo. External diagnostics such as blood tests, on the other hand, have none of these barriers and could greatly benefit from the increased precision of nanotechnology.
Cancer is currently difficult to detect in its early stages because cancer markers, such as proteins, DNA, mRNA and tumour cells, appear in low concentrations in blood.
As the director of the Australian Research Council Industrial Transformation Hub, Professor Jin has a $6.8 million grant to develop a diagnostic device that overcomes these issues. He is currently developing a new library of nanoparticles called super dots, tau dots, hyper dots and IDEAL dots, which he says could one day detect a single cancer cell among millions.
“The aim of this research is to develop a rapid screening test method so that we can quickly identify a very small number of cancer cells from the blood or urine,” says Professor Jin. “We are trying to find a needle in a haystack.”
In the future, for example, a handheld device may be able to analyse urine samples and detect cancer recurrences, says Professor Jin.
“I can see in the future that GPs might be able … to take blood or urine in their clinic and we will design tests with electrochemical nanoparticle sensors where they get a quick result,” adds Dr Phillips.
Many current blood and urine tests rely on organic dyes and fluorescent proteins. Super dots, which are a combination of rare earth compounds in crystal host such as calcium fluoride and sodium yttrium fluoride, have two main advantages over these materials.
Firstly, super dots have a large surface area to volume ratio, which means there is plenty of room to attach multiple molecules to optimise affinity for cancer markers.
Secondly, super dots can emit light at a greater range of wavelengths than conventional dyes, ranging from the ultraviolet, through the visible, and into the infrared spectra (300nm to 2000nm). The natural fluorescence of biological materials tends to interfere with the signal from traditional dyes.
But properties of super dots can be precisely controlled such that their emissions do not overlap with the background fluorescence, which makes their signal much clearer. The excited state of super dots also lasts much longer than traditional dyes, giving them stronger diagnostic properties.
Imaging
Current contrast agents, such as gadolinium or radionuclides for MRI, produce only limited resolution, making it difficult to detect early metastatic spread. Binding a higher density of these contrast agents to a single targeted nanoparticle can create a better snapshot of tumour tissue – bringing nanotechnology a step closer to the goal of theranostics.
For instance, last year researchers from CBNS combined MRI contrast agents with copolymer nanoparticles to create imaging probes in collaboration with ANSTO and the Memorial Sloan Kettering Cancer Centre in New York. The centre also developed a nanoparticle system to increase the detection of glioblastoma multiforme, the aggressive primary brain tumour.
Conventional imaging agents exploit the “leaky” physiology of blood vessels that supply tumours in order to passively accumulate inside them. Tumour vasculature is highly disorganised, and there are large gaps between endothelial cells relative to normal blood vessels. Imaging agents can pass from the bloodstream through these gaps into the tumour and are not speedily removed, as tumours often impair lymphatic drainage.
Targeted nanoparticles also use this permeability to build up in the tumour. But they go one step further by actively seeking out certain tissue types and then precisely targeting telltale biomarkers on the surface of individual cancer cells.
The CBNS team is now working on a nanoparticle that can respond ‘intelligently’ to the micro-environment it finds itself in. In the future, changes in the local biochemistry, such as a drop in pH or a change in tumour temperature, could trigger changes to the nanoparticle, increasing the contrast in precisely the right location – and maybe one day delivering drugs at the same time.
“The main advantage of a nanoparticle over the current technology is you can have it as a platform for multiple things so you can attach the actual drugs as well as the actual imaging agents,” says Professor Davis, who is involved in many of these projects at CBNS.
Professor Jin’s super dots are also strong candidates for alternative imaging agents. In fact, his lab won a Eureka Prize in 2015 for the development of super dot technology that could light up tumour sites from inside the body. Like other medical imaging chemicals, super dots produce electromagnetic signals that can transmit through thick tissue. But instead of using x-rays, gamma rays, radio waves or ultrasonic waves, super dots emit near-infrared light when stimulated with infrared light.
In the pipeline
Nano-sized gene therapy carriers hold potential as the ‘magic bullets’ for some of the most lethal cancers – but researchers still have a long way to go before this becomes a reality.
Dr Phillips’ team at UNSW is working on nanoparticle gene silencing systems for pancreatic cancer, for which there is little effective non-surgical treatment.
The research, published in Biomacromolecules in August, showed that a custom-built polymer in the shape of a star could successfully deliver short-interfering RNA (siRNA) to tumour cells, thereby interrupting the expression of the cancer-promoting protein ßIII-tubulin. The side arms of the star polymer can be manipulated to target specific cell types and increase stability.
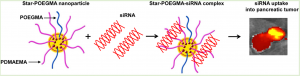
Mice treated with this star polymer complex had an 80% reduction in gene expression for ßIII-tubulin and an astounding 50% reduction in tumour growth.
The technique has received a lot of attention as a therapeutic target, due to ßIII-tubulin’s role in mitosis and association with low survival in cancer patients.
“Like many genes important in promoting cancer, [the gene for ßIII-tubulin] is undruggable because its structure is very difficult to design specific pharmacological inhibitors against,” says Dr Phillips.
Silencing the gene using this new RNA technique is more promising but is also quite difficult to achieve, as siRNA is degraded within the circulatory system without a protective coating. This is where nanoparticles come in.
“We built this [star polymer] from the ground up with some chemistry collaborators of ours to actually deliver
a short-interfering RNA into the pancreatic tumours to inhibit this gene,” says Dr Phillips.
Star-shaped polymers tend to remain small even when extra chemicals are added to them, as their “arms” wrap around the additional molecules.
Nanoparticles in this size range are capable of passing through the very thick scar tissue, which blocks conventional therapies from reaching pancreatic tumours.
“So we can overcome the restriction in the vessels by having this small system to get through this barrier – that’s a big breakthrough,” Dr Phillips says.
“Not only that; you could use this system to deliver any RNA inhibitor for any gene that you wanted to that was tumour promoting.”
Dr Phillips was also involved in a similar project for lung cancer, alongside lead researcher Professor Maria Kavallaris, the co-director of the Australian Centre for NanoMedicine at UNSW.
In 2015 their research, published in Oncotarget, found that a dendrimer nanoparticle system could reduce the spread of non-small cell lung cancer, which is the most common cause of cancer death worldwide.
The branched nature of the dendrimer used in this trial allowed for chemical conjugation with cell-targeting molecules.
“Nanomedicine is out there a lot in the literature,” says Professor Kavallaris. “It is not unusual for us to get contacted by patients saying, ‘Can I get access to this?’ It is important that GPs are aware of where this technology is moving because it is moving rapidly.”
For patients with terminal cancer, the false hope offered by nanomedicine can be shattering. “What has been quite devastating for me as a scientist this week is I’ve actually received over 200 emails from patients with pancreatic cancer wanting to test our drug obviously and willing to do anything because they are so desperate, they have nothing,” Dr Phillips says.
“And I’ve been really emotionally touched by this because you have to tell them that it could take seven to 10 years to get this exciting data from the mouse into the patient.”
CBNS researchers have been engineering targeted, porous silica microtubes for tumour-specific drug delivery. These nanoparticles are fabricated by electrochemical etching, which allows for extraordinary control over geometry and dimension.
The inner and outer surfaces can be functionalised for specific purposes, while the hollow tube can be stuffed with drugs. In 2015, CBNS researchers created microtubes filled with anticancer drug camptothecin, which killed 95% of neuroblastoma cells in vitro without touching healthy cells.
Putting it all together
All this research – from external diagnostics through to targeted drug delivery – form some of the pieces of the theranostics puzzle. But synthesising a ‘smart’ nanoparticle that can do everything all at once (locate, diagnose, penetrate cells and treat tumours) may prove to be much more difficult than simply adding more functions to existing nanoparticle systems.
“You need to make sure that when you make the system more complex that it’s efficient in each of its core designs,” says Dr Phillips. “If it is really good at diagnostics, you still need to make sure it has the same anti-cancer properties when you change its chemical composition. So there’s a lot of testing.”
The big challenge for theranostics is to outstrip standard chemotherapy in efficiency and in minimising side effects,
says Professor Davis.
“It is not enough to do what you can already do.”
Nanoparticles also have a number of drawbacks. For one thing, they suffer from the so-called size paradox. Only nanoparticles with diameters of 20nm or less can properly penetrate tumour cells in vivo. But as more elements are attached to nanoparticles, the size increases.
Even if the small size can be conserved, such small particles are rapidly cleared from the bloodstream, which again decreases the functionality. However, this might be overcome with multi-stage particles that break down over time as the particle passes through the body – not unlike a spacecraft discarding parts as it hurtles through the earth’s atmosphere.
The manufacture of nanoparticles is also a barrier. While the raw materials are often inexpensive, the characterisation is costly. “Characterisation is: ‘Have you got the right thing?; Is it reproducible?; Is it the right size?; Is it the right charge?; Is it the right shape?” explains Professor Kavallaris.
“One of the other things people are really aware of is nanotoxicology,” says Professor Davis. Nanomaterials have the potential to be very toxic because they are so small and can easily pass through human cell membranes.
“The surface of the nanoparticle is huge compared to the size and there you have a lot of interactions between the nanomaterial surface with proteins,” says Professor Jin.
These complex interactions give nanomaterials unique toxicology.
“And if you think about it, that is what you are exploiting in cancer treatment to some extent because most of the cancer treatments are toxic molecules by definition,” says Professor Davis.
Clinical trials need to ensure that nanoparticles are fully excreted rather than accumulating in the body. “And if they do biodegrade in any sort of way then we really need to know what all their breakdown products are and know that they’re safe,” says Professor Davis. “Those are fairly major challenges.”
Despite these obstacles, the research on theranostics is charging forward. Last year CBNS researchers designed theranostics for colon cancer in collaboration with International Medical University Malaysia. These orally-delivered nanoparticles pass through the digestive tract, delivering drugs while fluorescent imaging is used to track their progress.
“It’s really not pie in the sky anymore,” says Dr Phillips.