Thyroid cancer has excellent prognosis for cure, but management must be individualised, writes Dr Veronica Preda and Dr Diana Learoy
Australia has witnessed a steep rise in the incidence of differentiated thyroid cancer diagnosis over the last 30 years.
Most new cancers are early-stage cancers, and women outnumber men with a peak incidence around ages 45-50 years.
Some authors claim there is an epidemic of over-diagnosis caused by frequent use of neck ultrasound and “too-early” biopsy of thyroid nodules.
More than 90% of thyroid cancer patients achieve long-term remission but there are still many patients who develop stage-four disease which may be fatal.
Evidence-based guidelines from the American Thyroid Association have improved the old “one size fits all” approach to a tailored, individualised strategy where unnecessary treatments are minimised. There has been progress in both imaging and management of advanced thyroid cancers and systemic tyrosine kinase inhibitor (TKI) therapy is now available on the PBS for those who are refractory to radioactive iodine ablation.
DIAGNOSIS
Thyroid function testing is frequently performed in general practice as part of routine health screening. Patients with a past history or strong family history of thyroid disease may warrant TSH screening, but the diagnosis of any thyroid disease (especially cancer) requires clinical examination of the neck and cervical lymph nodes.
If there is thyroid asymmetry or a palpable neck mass, then neck ultrasound is warranted. A strong family history of nodular disease or thyroid cancer, or a past history of neck irradiation may also warrant neck ultrasound in the absence of signs or symptoms.
Thyroid nodules are highly prevalent in the general population (>50% of patients over 50 years) and commonly impalpable.
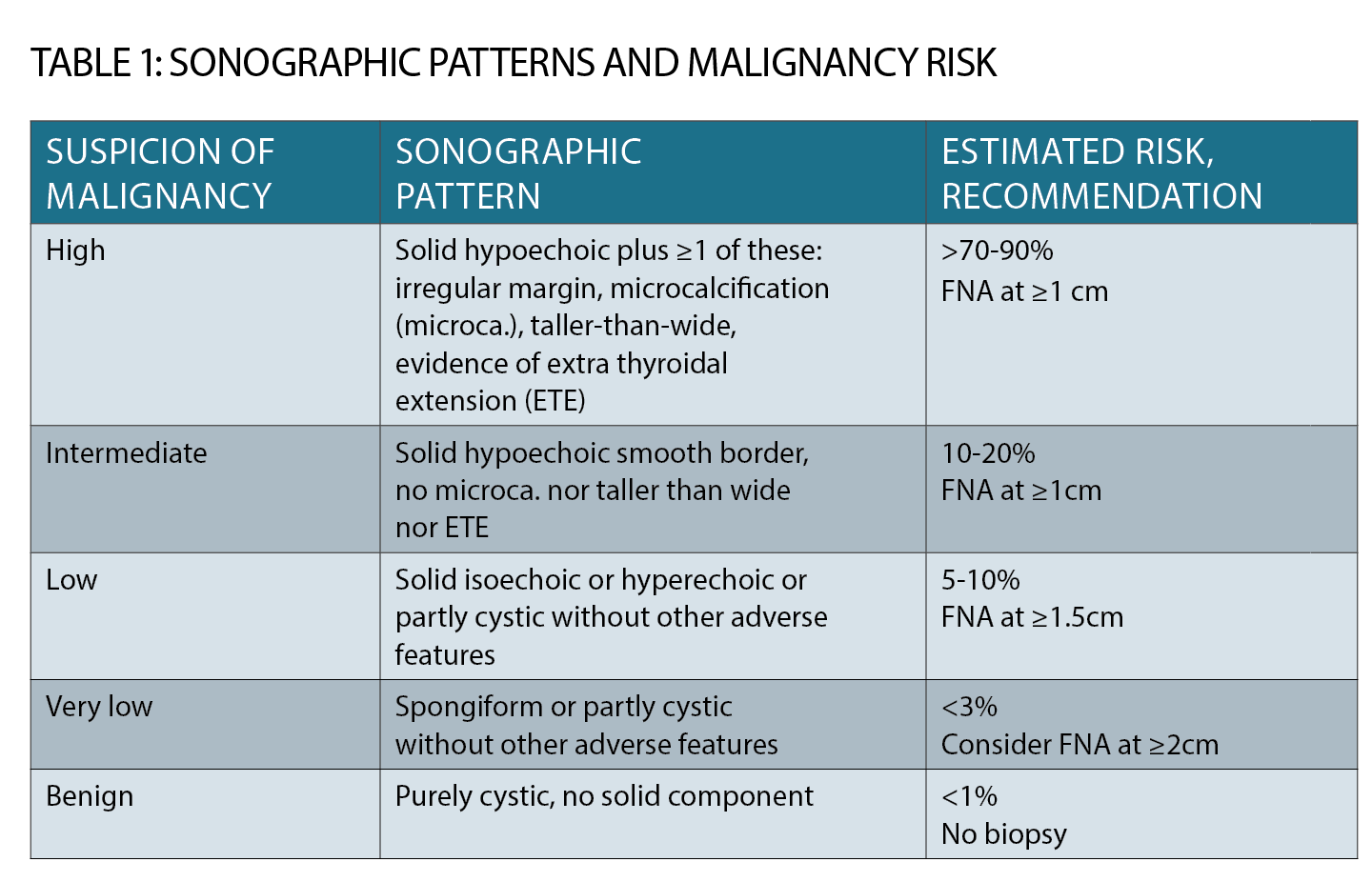
The decision to perform fine needle biopsy is now based on ultrasound criteria (See table 1, page 33). Size and nodule growth are considered, along with “worrying” features such as punctate micro-calcification, hypoechogenicity and irregular margins.
A pure cyst or a stable “spongiform” nodule may simply be monitored rather than biopsied.
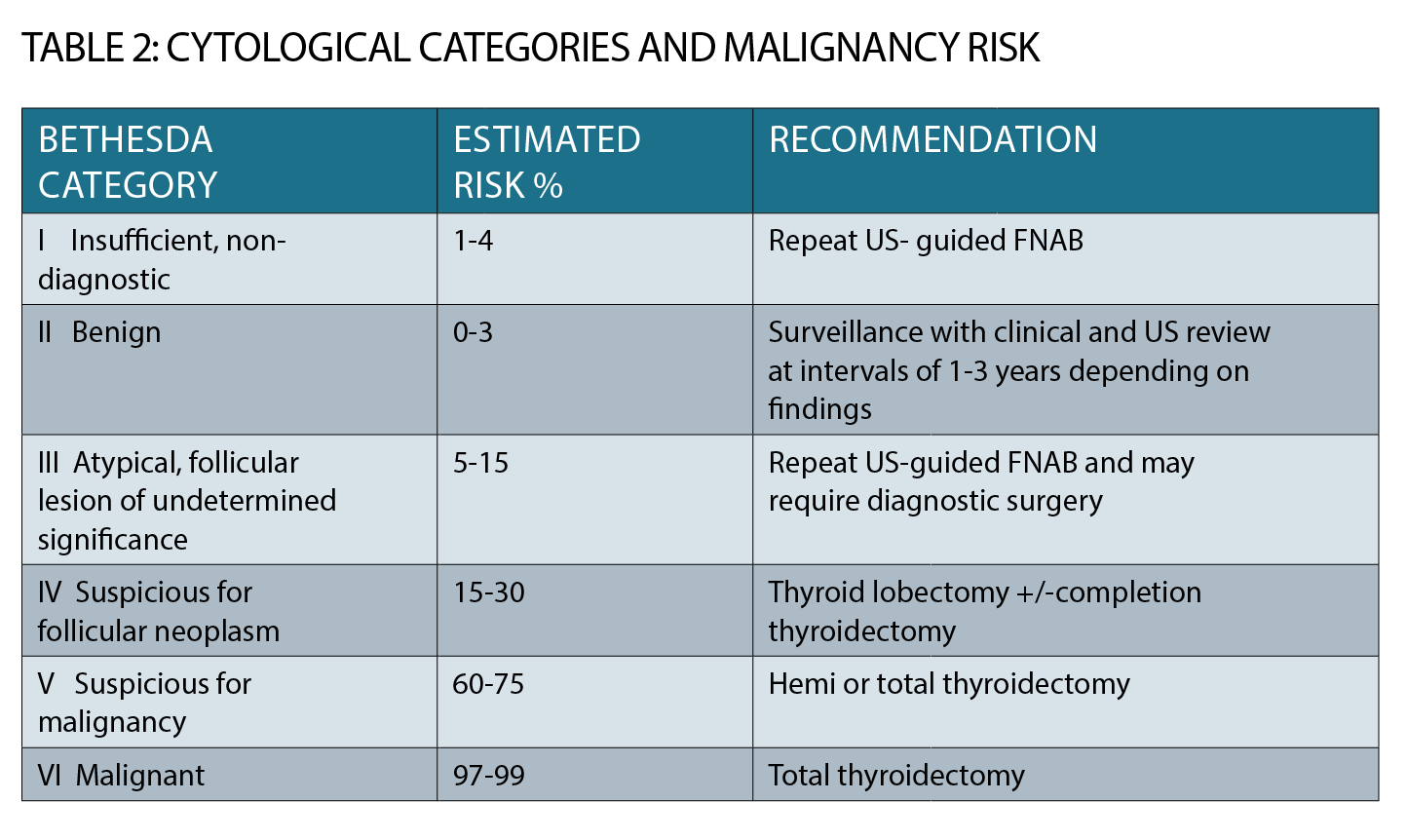
Most fine needle aspiration biopsies (FNAB) are now done under ultrasound guidance by expert radiologists and cytology is reported according to the Bethesda classification which gives six categories (See table 2, page 34).
Category 1 cytology (insufficient) may necessitate a repeat FNAB; category 2 is benign and does not require surgery although ongoing clinical and ultrasound monitoring will be required.
Management of Bethesda categories 3 to 6 is complex, beyond the scope of this article and requires specialist referral. Incidental “PET-avid” thyroid nodules are now identified on approximately 2.5% of FDG/PET scans and more than 30% of these are cancers.
Thyroid cancer is found in approximately 10% of biopsied nodules and there is now a trend towards less-aggressive management of low-risk cancers.
Papillary thyroid cancer is the commonest type (75%) and is often multifocal and spreads early to local cervical lymph nodes, but despite this it has a very good prognosis for long-term cure (>95% 10 year survival), particularly when diagnosed under age 55 years.
Other types are follicular thyroid cancer (FTC, 15%), which can exhibit distant spread through vascular invasion, and the rare, but aggressive, poorly differentiated subtypes including anaplastic thyroid cancer. Medullary thyroid cancer (MTC) is rare (5%), arising from neuroendocrine calcitonin-secreting C cells, and sometimes inherited as part of the autosomal dominant multiple endocrine neoplasia (MEN) type 2 in patients with germline mutations in the RET oncogene. It is treated differently to differentiated thyroid cancer.
SURGERY
Surgery for differentiated thyroid cancer can be a diagnostic nodulectomy/hemithyroidectomy (with or without completion thyroidectomy), or total thyroidectomy (with or without central or lateral lymph node dissection) and these decisions are based on clinical impression, cytology and sometimes even pre-operative staging with anatomical and functional scans.
There is a trend now to less-aggressive surgery in low-risk differentiated thyroid cancer cases and hemi or total thyroidectomy alone may suffice without the need for radioactive iodine ablation.
The risks of surgery are low when performed in high-volume centres, with the main risks being recurrent laryngeal nerve damage (causing permanent voice change) and parathyroid devascularisation causing permanent hypocalcaemia. Both risks are <1% in high volume surgical centres.
Formal surgical histopathology then guides the ongoing management along with dynamic risk re-stratification based on the patient’s clinical response. The ATA guidelines regularly update the categories of risk (currently divided into low, intermediate and high) and genetic profiling (e.g. somatic BRAF mutation) is now mentioned in risk categorisation and testing available in some pathology labs.
RADIOIODINE ABLATION
The decision to ablate the thyroid bed post-operatively with radioactive iodine ablation along with the dose chosen are now individualised decisions based on other risks such as age, medical history, gender, and the size and histopathology.
Small papillary thyroid cancers with no nodal spread may be managed with surgery alone.
Radioactive iodine remnant ablation remains useful where there is known residual microscopic disease and the post-ablation scan will clarify whether this disease is iodine avid and thus likely to respond to the radioactive iodine dose.
Radioactive iodine doses are now routinely lower than in the past, and level 1 evidence supports doses as low as 1GBQ (25 mCi) for low-risk disease.
A low-iodine diet must be adopted for at least one week prior to radioactive iodine ablation
SERUM THYROGLOBULIN TESTING
Endocrinologists can monitor the serum thyroglobulin as an accurate tumor marker (ideally undetectable).
GPs need to be aware that thyroid cancer patients must have regular monitoring of both thyroglobulin and also thyroglobulin antibodies. If antibodies are positive, then serum thyroglobulin is less accurate as it is falsely lowered.
Thyroglobulin antibodies tend to disappear with time when therapies are effective in clearing residual thyroid tissue.
RECURRENCE
Patients with bulky nodal involvement may respond well to initial therapies but later nodal recurrences may necessitate further cervical lymph node surgical clearance years down the track.
Papillary thyroid cancer is associated with a recurrence risk as high as 30% in intermediate risk groups. FDG/PET scanning and high-resolution neck ultrasound have greatly improved the sensitivity of detection of cervical nodal disease.
Iodine surveillance scans are rarely used these days in the routine follow up of low-risk papillary thyroid cancer patients.
TSH LEVELS
Large doses of thyroxine replacement, (enough to suppress TSH) were routine in the past but the guidelines now specify that “cured” differentiated thyroid cancer patients should have lower thyroxine doses to allow TSH normalisation, especially in those at high risk of morbidities such as bone loss and cardiac arrythmia.
However, GPs should also note that in patients with advanced or persistent thyroid cancer, suppression of TSH below 0.1mIU/L is important in controlling tumour growth so thyroxine dose reductions should only be done in conjunction with specialists.
ADAVANCED DISEASE
Radioactive iodine ablation remains a very effective therapy for advanced metastatic disease (if it is radioactive iodine avid) and occasionally young patients with stage 4 disease where radioactive iodine avidity remains good can be cured. Cure is less likely in older stage 4 patients (diagnosed over age 55) and in some poorly differentiated histological subtypes (e.g. insular thyroid cancer) especially if radioactive iodine avidity is low.
We are now moving into the “genetic era”, albeit behind medical oncology in terms of targeted therapies. Thyroid cancer genetics knowledge is expanding rapidly and management decisions may ultimately be based on genetic profiling of tumours.
The somatic V600E BRAF mutation that is observed in other cancers such as melanoma has been widely studied in thyroid cancer where it is ubiquitous (60 to 70%) but not yet routinely tested in all Australian pathology laboratories.
Its presence may guide clinical management in some situations (e.g. intermediate-risk cases with bulky nodal disease) but clarification of its role remains a work in progress.
Systemic therapies for progressive symptomatic radioactive iodine refractory thyroid cancer have been trialled in Australia and Lenvatinib is now available on the PBS for these cases.
Side effects are seen in more than 50% of patients and Lenvatinib should only be prescribed by specialist endocrinologists or oncologists familiar with its dosing and potential toxicities.
Fatigue, anorexia, hypertension, palmar/plantar desquammation and proteinuria are common toxicities.
PREGNANCY
Management around the time of pregnancy is an important issue as differentiated thyroid cancer is not uncommon in women of child bearing age.
Sometimes suppressive doses of thyroxine are adjusted to normalise TSH to improve fertility in women who are keen to conceive. The thyroxine dose requirement will rise significantly after conception in thyroidectomised women and patients need to be warned to automatically increase their dose as soon as pregnancy is confirmed (by 20 to 30%).
TSH testing is performed early in the first trimester if possible and four to six weekly thereafter until the third trimester. Pre-pregnancy doses of thyroxine are resumed post-partum.
It is important to avoid maternal hypothyroidism in the first trimester when the fetal thyroid is not fully developed but when fetal brain development is crucial.
There is concern with regards to the risks of pregnancy in patients with residual differentiated thyroid cancer, but there is no clear evidence that tumour progression is caused by pregnancy (in contrast to the situation with hormonally responsive breast cancer).
Patients should avoid pregnancy for at least six months (ideally 12 months) after radioactive iodine ablation.
Fertility rates are likely not affected by radioactive iodine dosing in the distant past (assuming the cumulative dose is low) although there may be a small effect on earlier induction of menopause.
In men there is a transient reduction in sperm count after radioactive iodine which is usually reversible and sperm banking is not routinely recommended unless it is felt that very large total doses of therapeutic RAI will be required.
CONCLUSION
Thyroid cancer is a disease of all ages, but particularly one of middle-aged women.
Overall it has an excellent prognosis for cure, but management must be individualised and specialist care is recommended especially around the time
of pregnancy.
Serum thyroglobulin is a useful tumour marker which must be monitored along with thyroglobulin antibodies, and TSH levels.
TSH targets for thyroxine therapy are also individualised and TSH suppression is an important goal of therapy in those with persistent or advanced thyroid cancer.
New systemic therapies for advanced disease are now available in Australia.
Veronica A Preda, MBBS Hons FRACP PhD, is based at the Department of Endocrinology, Macquarie University Hospital, Sydney
Diana L Learoyd, MBBS Hons FRACP PhD, is based at the Sydney Medical School, Northern Campus, Royal North Shore Hospital, Sydney
References:
1. Haugen BR, Alexander EK, Bible KC et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 26(1) 2016 DOI:10:1089/thy.2015.0020.
2. Preda VA, Preda, TC and Learoyd DL. Thyroid Cancer; an update on diagnosis and management. Endocrinology Today Apr 2017; 6(2):20-25.