Many people are taking glucosamine for their osteoarthritis. But do they really need to stop in light of new safety warnings?
The Australian Rheumatology Association recently warned people not to take the supplement glucosamine for their osteoarthritis due to possible allergic side-effects.
So what’s the evidence behind this latest advice?
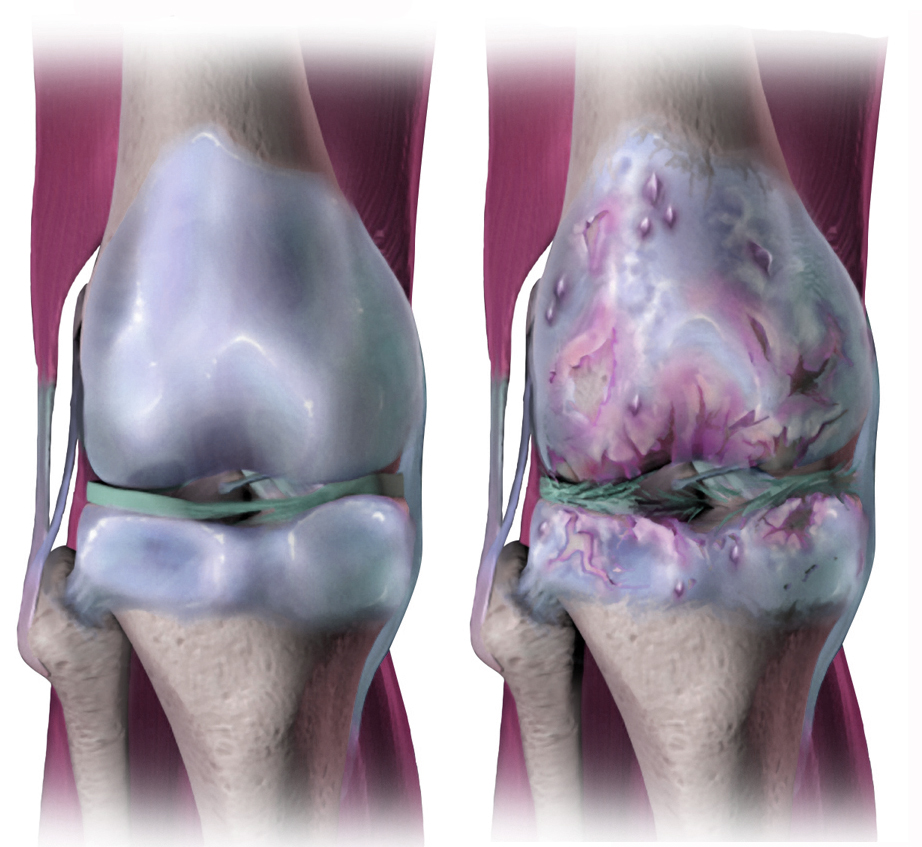
For years, glucosamine has been marketed as a treatment for osteoarthritis, which can occur when the protective cartilage in the joints wears down over time.
This is despite conflicting evidence on whether the supplement works. Yet many patients may buy glucosamine, presuming that even if it doesn’t help, at least it’s “natural” and so won’t do any harm.
Read more: Arthritis isn’t just a condition affecting older people, it likely starts much earlier
But an Australian study, which has been online since last year and was cited in one of last week’s media reports, has given us more information about glucosamine’s safety.
The study found hundreds of allergic reactions to glucosamine have been reported to Australia’s medicines watchdog, the Therapeutic Goods Administration (TGA).
What is glucosamine?
Glucosamine is a naturally occurring substance the body uses to help build joint tissue, such as cartilage and tendons. In a supplement, the glucosamine can be made from the shells of prawns and other crustaceans, or it can be made synthetically in a factory.
Whether it works to manage osteoarthritis seems open to debate. The most recent evidence suggests little to no clinical benefit.
Read more: Science or Snake Oil: is glucosamine good for joints?
But advice to GPs about how to treat osteoarthritis says the issue isn’t just confined to glucosamine.
When the Royal Australian College of General Practitioners looked at about 62 other medicines and possible treatments for osteoarthritis of the knee and hip (which include registered drugs and complementary medicines), none were backed by high-quality evidence to say they worked. Most of the evidence was based on low- or very low-quality studies.
Is glucosamine really as dangerous as people say?
The Australia study found 336 cases of side-effects to glucosamine (and to another supplement used for osteoarthritis called chondroitin) were reported to the TGA over 11 years. Of these, 263 cases were allergies, which ranged from mild to severe.
We don’t know if these reactions included those from people with a known allergy to seafood or sulfur, as these would increase their risk of a reaction to glucosamine (glucosamine can come in different formulations, including glucosamine sulfate).
But a large percentage of people take glucosamine daily in Australia, with no ill effects. The cases reported to the TGA amount to just 30 people a year, with 16% of allergic reactions considered severe.
Beyond allergic reactions, there are other safety concerns about glucosamine.
For instance, if you are taking glucosamine and a medicine that thins blood (such as warfarin after a stroke), this can increase risk of bleeding.
Read more: Weekly Dose: Warfarin, the blood-thinner that’s still used as a rat killer
Glucosamine supplements have also been implicated in chronic liver disease and in worsening underlying asthma. Some patients may also experience digestive symptoms such as heartburn.
The risks of other side-effects seem unclear, including whether it raises blood glucose levels in people with or without diabetes.
Conflicting advice
While the Australian Rheumatology Association has warned people to stop taking glucosamine, other advice is not so clear-cut.
Arthritis Australia reports glucosamine is a relatively safe treatment option for people with osteoarthritis and has relatively few side-effects compared with traditional medicines.
And the guidelines for GPs on how to manage osteoarthritis of the knee and hip makes a “conditional” recommendation not to use it, based on uncertainty over the balance of harms with potential benefit.
Authors
Associate Professor | Program Director, Undergraduate Pharmacy, University of Sydney
Lecturer (Complementary Medicines) Sydney Pharmacy School, Faculty of Medicine and Health, University of Sydney
Disclosure
Associate Professor Wheate in the past has received funding from the ACT Cancer Council, Tenovus Scotland, Medical Research Scotland, Scottish Crucible, and the Scottish Universities Life Sciences Alliance. He is Fellow of the Royal Australian Chemical Institute and a member of the Australasian Pharmaceutical Science Association. Nial is also a director of the medicinal cannabis company Canngea Pty Ltd and a Standards Australia committee member for sunscreen agents.
Joanna Harnett has received funding in the past from, Sigma Pty Ltd (operating as Orphan Australia) and Bioceuticals Pty Ltd in the past to conduct probiotic research. She is a board member of the Australasian Integrative Medicine Association.
This article is republished from The Conversation under a Creative Commons licence. Read the original article