Remdesivir can now considered for use outside the clinical trial setting to treat COVID-19, according to new advice from the National COVID-19 Clinical Evidence Taskforce.
Thanks for joining us on The Medical Republic‘s live COVID-19 blog.
Wishing you a safe, healthy and COVID-19-free long weekend.
The latest
- Afternoon update: Conditional recommendation for remdesivir use outside clinical trials, AHPPC decides now is the time to express its concern about protests, and the latest TMR podcast is live.
- Morning update: Retractions follow data concerns over HCQ paper, WA lifts regional travel restrictions, and it’s Crazy Socks For Docs day.
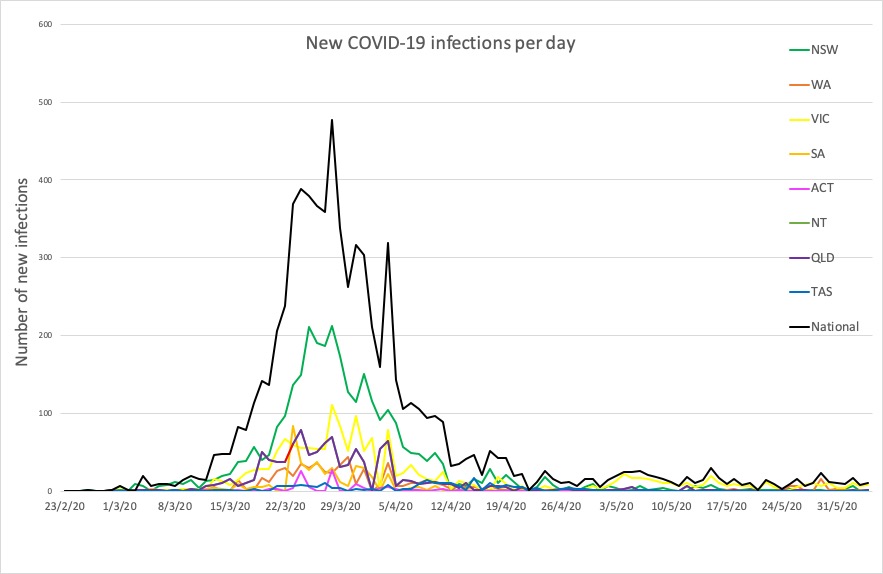
- Today’s updated COVID-19 infection figures for Australia shows zero new infections for NSW.
- Remdesivir can now be considered for use outside the clinical trial setting to treat COVID-19, according to new advice from the National COVID-19 Clinical Evidence Taskforce.
This multi-organisational taskforce is engaged in a real-time reviewing of any and all new evidence emerging about COVID-19 to update its ‘living guidelines’ on the disease.
This latest conditional recommendation acknowledged that there is evidence from two randomised clinical trials of a small net benefit in terms of decreased time to recovery associated with remdesivir, and that it has an acceptable safety profile.
The taskforce stressed that it was still preferable that the drug be given within the context of a clinical trial, but this was no longer a requirement. - Just in time for the protests against Aboriginal deaths in custody and in support of Black Lives Matter – but, oddly enough, weeks too late for the anti-5G, anti-vaccine, anti-Bill-Gates, COVID-19-conspiracy tin-foil-hat-wearing brigade protests – the Australian Health Protection Principal Committee has issued a caution about large gatherings and the risk of COVID-19 resurgence.
“These large protests encourage mixing of people in the population who are not part of usual social networks,” the AHPPC statement pronounced, recognising that these protests are likely to bring Australians from all walks of life out into the streets. - We’ve had a few interesting COVID-19 stories crop up this week – and we’ve got Bianca Nogrady, our live blogger, here to tell us all about it on TMR‘s weekly podcast
- Both The Lancet and the New England Journal of Medicine COVID-19 papers that used data from Surgisphere have been retracted overnight. The retractions followed revelations from The Guardian’s investigation, and concerns expressed by experts and journal editors, about the integrity of the international hospital registry data provided by the data analytics company.
That data underpinned a study suggesting hydroxychloroquine and chloroquine – alone or in combination with a macrolide – were associated with higher in-hospital mortality and new ventricular arrhythmias in patients with COVID-19, which prompted WHO to suspend its hydroxychloroquine clinical trial. That trial has now resumed, and other hydroxychloroquine studies – including one in Australia – are also continuing. - Western Australia is lifting its restrictions on travel into regional areas but the restrictions remain on travel to remote Aboriginal communities and into the Kimberley region.
- It’s Crazy Socks For Docs day, to raise awareness of mental health in healthcare professionals – something that’s always important but particularly so during a global pandemic and SNAFU.
Here are some of the finer examples of fancy foot-frippery you all have been toting this morning (and for more check out #crazysocks4docs on Twitter):
Here’s my effort for #CrazySocks4Docs today. @gdtoogood got the idea after he turned up for work one day wearing odd coloured socks. His hospital colleagues whispered about his ‘failure’ to wearing matching socks. He started this initiative to make it ok for a doctor to not be ok pic.twitter.com/igZtRtZMuN
— RACGP President (@RACGPPresident) June 4, 2020
Family has joined the conversation and we are talking about mental health and making safe spaces #CrazySocks4Docs pic.twitter.com/grGpurXcaf
— Dr Kerrie Aust ????to?? (@Kezpower1) June 4, 2020
Please look after yourselves and each other. It’s OK not to be OK. #CrazySocks4Docs pic.twitter.com/KmSXUYKAG3
— ChrisMoyAMASA (@ChrisMoyAMASA) June 4, 2020
#CrazySocks4Docs for all of our colleagues .. we are in this together ?@RACGP? ?@WoncaWorld? pic.twitter.com/CPgBZxN7tc
— Gibraun Brijmohan (@DrGibraun) June 5, 2020
9.30am, 5 June
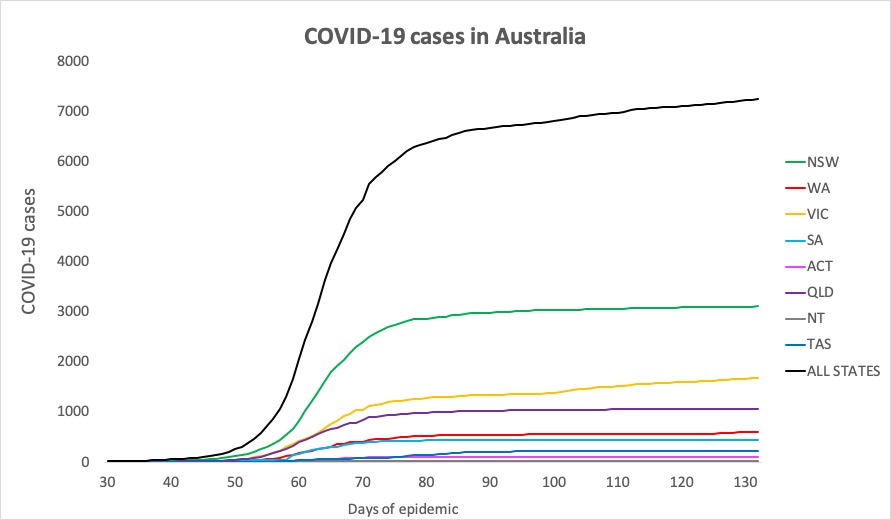
- No major developments in Australia’s COVID-19 infection figures this morning; no news is good news. Here’s the count to 9pm yesterday:
National – 7240, with 102 deaths and 6664 recovered
ACT – 107
NSW – 3106
NT – 29
QLD – 1060
SA – 440
TAS – 228
VIC – 1678
WA – 592
Disclaimer: The content on the Medical Republic COVID-19 blog is independently created by Medical Republic without input from Boehringer Ingelheim Pty Ltd. The views, information, or opinions expressed on the Medical Republic COVID-19 blog are Medical Republic’s own and do not necessarily represent those of Boehringer Ingelheim Pty Ltd. Boehringer Ingelheim Pty Ltd is not responsible for and does not verify the accuracy of any content on the Medical Republic COVID-19 blog.


