A small cohort study suggests individuals with Down syndrome may be at significantly greater risk of contracting COVID-19 and experiencing more severe disease, according to a paper published in the non-peer-review preprint server MedRxiv.
Thanks for joining us on The Medical Republic‘s live COVID-19 blog.
If you have any tips, comments or feedback, get in touch at bianca@biancanogrady.com.
The latest
- Afternoon update: Australian HCQ trial to go ahead, Down syndrome may increase risk of severe COVID-19, and study finds no benefit from convalescent plasma transfusions.
- Morning update: Reassurance on PCR tests after QLD false-positive, more doubts cast on company behind hydroxychloroquine study, and psychological distress widespread in adults during COVID-19.
- Today’s updated COVID-19 infection figures for Australia shows zero new infections for NSW.
- A small cohort study suggests individuals with Down syndrome may be at significantly greater risk of contracting COVID-19 and experiencing more severe disease, according to a paper published in the non-peer-review preprint server MedRxiv.
A retrospective, single-centre study of 4,615 patients hospitalised with COVID-19 in New York identified six individuals with Down syndrome, which they estimated as representing a nearly nine-fold higher risk of hospitalisation compared to individuals without Down syndrome from the same age range.
The median age of those with Down syndrome was also significantly lower than the median age of COVID-19 patients without Down syndrome. The authors also compared disease course and outcomes between the six Down syndrome patients and 30 controls matched for age, sex and ethnicity, and found two-thirds of the Down syndrome patients progressed to sepsis compared to 20% of the control group.
“The increased incidence of sepsis in our Down syndrome cohort is particularly relevant given studies that patients with Down syndrome are at higher risk of mortality secondary to sepsis than septic controls,” they wrote, calling for greater focus on prevention and treatment of COVID-19 in individuals with Down syndrome. - The Australian COVID-19 hydroxychloroquine study is continuing despite the recent controversies and contentious data on outcomes from treatment with the antimalarial drug.
The randomised, controlled AustralaSian COVID-19 Trial (ASCOT – excellent acronym-wrangling effort) will now restart patient recruitment, according to a statement from ASCOT principal investigator, Associate Professor Steven Tong from Royal Melbourne Hospital and the Doherty Institute.
“ASCOT is also an adaptive trial, which means that we are able to stop the trial if one of the treatments gives us enough evidence that it will work, or drop any treatment that looks like it will not work before the trial would typically finish,” Professor Tong said. - A study of convalescent plasma transfusions has found no significant improvements in outcomes in patients with severe or life-threatening COVID-19 infection.
Published in JAMA, the study in 103 patients from seven medical centres in Wuhan found no statistically significant differences in clinical improvement or mortality within 28 days among patients who were given transfusions of plasma from recovered COVID-19 patients in addition to standard care, and those who were treated with standard care alone.
The study was terminated early due to difficulties in recruiting patients after the outbreak eased in Wuhan.
Some previous studies have shown a benefit from convalescent plasma transfusions. Commenting on earlier findings, the authors said that most studies so far have had a lack of standardisation of patients and procedures in terms of donor selection and the level antibodies in the plasma units.
“This may explain the varied therapeutic effects seen across a variety of diseases or even across patients with the same disease,” they wrote.
- From TMR’s Penny Durham: The case of Nathan Turner in Blackwater, Queensland, who was initially believed to have died from COVID-19 but tested negative at autopsy, has prompted reassurance that the PCR test is in fact highly accurate at detecting SARS-CoV-2 infection.
Professor Ivo Mueller from the Population Health and Immunity Division at The Walter and Eliza Hall Institute in Melbourne told a briefing this morning that false negatives were far more likely than false positives.
Though he couldn’t comment specifically on that case, he said a false positive could be the result of contamination or the highly improbable accident of another agent displaying the same genetic sequence as one of the targets of the PCR test.
There was also the fact of declining positive predictive value when actual case numbers are low – the fewer true positives in a tested population, the higher the likelihood of a false positive.
The testing system, which included multiple confirmatory tests and quality control, was “generally very robust”, so the case of Mr Turner – which caused significant disruption in his town – was unfortunate.
In an infected patient with high viral load, he said, the test was very sensitive and specific – it would not pick up any other coronavirus.
But results could be unreliable in the early stages of infection where viraemia was low, and later when the patient had almost cleared the virus.
“It’s rare that the PCR fails, it’s usually that there was no virus in the sample.”
Technical failure of the test would be picked up immediately in the quality control stage of the process.
And while it was possible for genetic material to linger after the virus was cleared and test positive, Professor Mueller said, such debris did not usually hang around for long.
How important the issue of false negatives was depended crucially on how infectious a person with undetectable virus was, he said. This was still unknown, but evidence was mounting that the fewer the symptoms, the lower the infectivity.
Sometimes a patient with pneumonia will test negative on a swab while showing COVID-19 changes on a CT scan – in which case their sputum would likely be positive – but in those cases a diagnosis would never rely on PCR alone. - The scandal surrounding the international hospital dataset that has underpinned two major COVID-19 papers has deepened, with revelations from The Guardian that the company behind it – Surgisphere – is a little-known US health analytics company whose chief executive has been named in three medical malpractice suits.
As more questions are raised over the integrity of Surgisphere’s data, the World Health Organization has announced it will restart its hydroxychloroquine study, which it terminated after a study based on Surgisphere data, published in The Lancet, suggested increased in-hospital mortality in COVID-19 patients. - Significantly more adults are reporting serious psychological distress during the COVID-19 pandemic, according to a study published in JAMA.
An online survey of 1468 US adults found that in April this year, nearly 14% reported experiencing serious psychological distress, compared to just under 4% in April 2018. Nearly one in four adults aged 18-29 years reported symptoms of psychological distress, compared to 3.7% of young adults two years ago. Rates were also significantly higher among adults in lower-income households and among Hispanic adults.
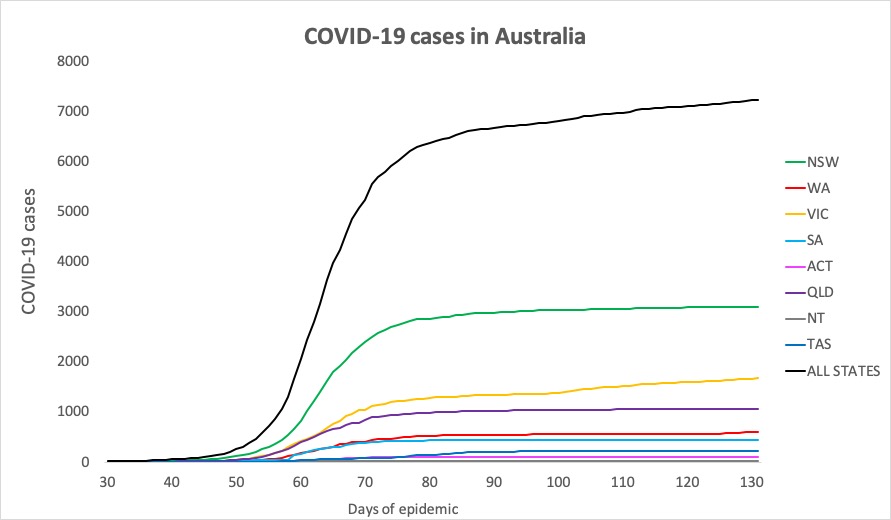
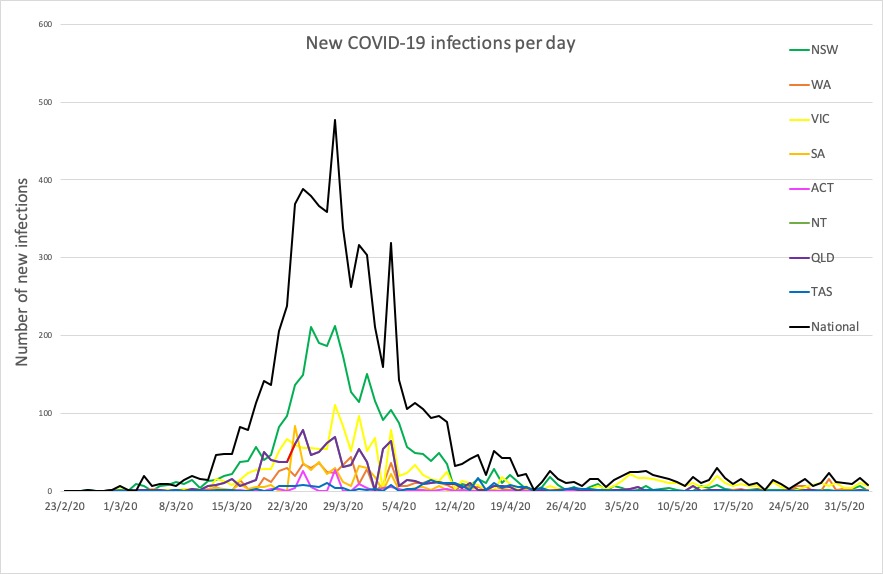
- New South Wales recorded not a single new infection in the 24 hours to 9pm yesterday, which is always cause for celebration. Here are the confirmed infection figures for around Australia:
National – 7229, with 102 deaths and 6640 recovered
ACT – 107
NSW – 3104
NT – 29
QLD – 1059
SA – 440
TAS – 228
VIC – 1670
WA – 592
Disclaimer: The content on the Medical Republic COVID-19 blog is independently created by Medical Republic without input from Boehringer Ingelheim Pty Ltd. The views, information, or opinions expressed on the Medical Republic COVID-19 blog are Medical Republic’s own and do not necessarily represent those of Boehringer Ingelheim Pty Ltd. Boehringer Ingelheim Pty Ltd is not responsible for and does not verify the accuracy of any content on the Medical Republic COVID-19 blog.