MBS has clarified the definitions of ‘chronic health condition’ and ‘immune compromised’ with respect to telehealth eligibility.
That’s it for today on The Medical Republic‘s live COVID-19 blog.
Thanks to our sponsor and supporter for funding this project with an independent grant, Boehringer Ingelheim.
The latest
- What to do with patients taking thiopurine during COVID-19 pandemic?
- MBS clarifies definitions of ‘chronic health condition’ and ‘immune compromised’ for telehealth.
- Wondering what the deal is with remdesivir? Here’s our attempt to distill the latest.
- Should patients taking thiopurine, which is associated with an increased risk of viral infection, stop their medication during the COVID-19 pandemic? The authors of a paper in the Medical Journal of Australia say no.
Thiopurines are immunosuppressant drugs used in oncology, immunology, and for the treatment of inflammatory bowel diseases such as Crohn’s disease. Previous research has linked them to serious viral infections, although the pathogens involved were all herpes viruses.
In their article, the authors examined whether the COVID-19 pandemic posed a risk to patients taking thiopurines, given this association and given the lack of information about the interaction between thiopurines and other coronaviruses such as SARS-Cov-2 and MERS.
They cited preliminary data from a COVID-19 database for inflammatory bowel disease which they said provided “cautious support” for the relative safety of these drugs.
They also pointed out that thiopurine withdrawal is associated with a significant 12-month relapse rate.
“Perhaps the best advice we can currently offer patients is that effective control of disease may carry less risk than poorly considered withdrawal of therapy,” they wrote.
- MBS has clarified the definitions of ‘chronic health condition’ and ‘immune compromised’ with respect to telehealth eligibility.
As posted on the RACGP’s website, Medicare defines a chronic condition as a medical condition that has been present or is likely to be present for at least six months, or is terminal. Immune compromised is essentially up to the treating doctor to define.
In both cases, when it comes to telehealth eligibility, the provider should document how the patient meets the criteria for chronic medical condition or immune compromised, and provide whatever appropriate documentation would be “generally accepted by the relevant profession as necessary to demonstrate eligibility”.
- If you’re a vampire who likes to bathe in the blood of virgins, why not throw some COVID-19 convalescent plasma in there and add some immunological benefits to your skincare and wellness regimen?
According to an ABC report, ANU researchers have discovered that the blood of recovered COVID-19 patients is being sold on the dark web – the internet’s seedy underbelly – alongside PPE and purported cures for the disease such as anti-malarial drugs.
The story came out as part of a report by the Australian Institute of Criminology into how cybercriminals are taking advantage of the pandemic. Clearly the denizens of the dark web have been reading the recent studies – with very small patient numbers – of convalescent plasma transfusions possibly shortening the duration and intensity of severe COVID-19.
- Meanwhile, as the US works itself into a lather about remdesivir or chloroquine or UV light or disinfectant, Australia’s Chief Scientist and other academics have warned that the community needs to be alert about claims of breakthrough treatments and sudden cures for COVID-19 and be wary of ‘fake news’.
In a report in the Guardian, Dr Alan Finkel said that while open sharing of data is important “with openness comes the need for the public to understand that the answers are not simple and that understanding is achieved through the weight of evidence from multiple trials.”
- If you didn’t get time to read this live blog over the past week but you’d like to hear the latest updates for GPs, we’ve got you covered! In this podcast episode, TMR‘s Felicity Nelson and Francine Crimmins are joined by blog editor Bianca Nogrady to give you a summary of what’s been happening.
- What the heck is happening with the antiviral remdesivir in COVID-19? If you’re getting whiplash from watching results ping from ‘promising’ to ‘dead in the water’ and back to ‘FDA-approved for COVID-19’, rest assured you’re not the only one.
The last 24 hours have seen the release or publication of results from not one but three remdesivir trials; one from China published in the Lancet, one international study sponsored by manufacturer Gilead and published by press release, and one from the National Institutes of Health also published by press release. There are also reports the FDA is considering approving the drug for COVID-19.
The first of these studies – a randomised, double-blind, placebo-controlled, multicentre trial in Hubei, China – was actually leaked a week ago but the results have just been published in the Lancet. This involved 237 patients admitted to hospital with radiologically confirmed pneumonia and oxygen saturation at 94% or less. It’s worth noting that this trial was terminated early, before it had recruited its original target number of patients.
The trial randomised patients in a 2:1 ratio, so twice as many received remdesivir as received placebo. Nearly one in five patients were also treated with the antivirals lopinavir–ritonavir.
The study found no significant differences between the two groups in time to clinical improvement or in mortality, despite a follow-up of 28 days. They also saw a much higher rate of discontinuation due to adverse events in the remdesivir group compared to the placebo group.
When the authors restricted the analysis to patients who were treated with remdesivir or placebo within 10 days of symptom onset, they did see a numerical (but not statistically significant) difference in favour of remdesivir in time to clinical improvement, in mortality and in duration of invasive mechanical ventilation.’ This prompted the authors to note that they weren’t able to adequately assess whether earlier treatment might have provided clinical benefit, because as the trial was enrolling the implementation of public health measures meant it was harder to recruit patients and those they did recruit tended to have more severe illness.
Now to the National Institutes of Allergy and Infectious Diseases study, which institute director – Dr Anthony Fauci – has just talked up while sitting in the Oval Office with Trump glowering nearby. Yes, he did describe remdesivir as the new standard of care against which all other potential COVID-19 treatments should be measured, and likened these results to the first days of antiretroviral therapy trials in the HIV/AIDS epidemic.
The results of the NIAID’s Adaptive COVID-19 Treatment Trial (ACTT) have not been published in full – not even on preprint servers – so we’re only given the scraps by press release. The trial is a randomised, placebo-controlled trial of ten daily infusions of remdesivir in 1063 patients with laboratory-confirmed COVID-19 and either chest signs on x-ray, oxygen saturation at or below 94%, need for supplemental oxygen or being mechanically ventilated – so similar to the Chinese study’s patient selection.
According to the NIAID press release, the preliminary analysis found a statistically significant 31% improvement in time to recovery with ten daily infusions of remdesivir compared to placebo, which translated to a median time to recovery of 11 days versus 14 days. There was also a non-significant trend towards improved mortality: 8% in the remdesivir group compared to 11.6% in the placebo group. It doesn’t talk about the median follow-up time, although the study description on the Clinical Trials Register says the primary outcome is time to recovery by day 29.
The press release is alarmingly light on details given that it also talks about the US FDA making the drug available to COVID-19 patients as quickly as possible.
And that leads us to the third study, sponsored by manufacturer Gilead and published by press release. This is the open-label, phase 3 SIMPLE trial in 200 patients treated with a five-day course and 197 treated with a 10-day course of remdesivir. There was no control or placebo group in this study, and patients were required to have either signs of pneumonia or reduced oxygen but not on mechanical ventilation.
Gilead’s analysis showed similar times to clinical improvement between the two treatment arms, and reported that more than half the patients in both groups were discharged from hospital within 14 days. However it also showed that patients who were treated earlier – within 10 days of symptom onset – were more likely to be discharged from hospital by day 14 compared to those who were treated after 10 days since symptom onset.
They did also show twice as many patients in the 10-day treatment group stopped the treatment because of adverse events compared to the 5-day trial.
There are now reports that the FDA is planning to issue emergency-use authorisation for remdesivir in COVID-19. - Wondering about science Twitter’s take on remdesivir? Here are a few comments:
I discussed here 3 studies on #remdesivir treatment for #COVID19.
1/ The double blind clinical trial on 237 patients in China
2/ The double blind clinical trial from the @NIH on 1063 patients
3/ 5 day vs 10 day treatment in a cohort of 397 patientsBottom line. No silver bullet https://t.co/kRq0ynoR8w
— Dr Gaetan Burgio, MD, PhD. (@GaetanBurgio) April 29, 2020
Maybe we could all wait to see the actual trial data and design before hyping this latest Remdesivir trial? Recent medical announcements from the White House don’t have a great track record for reliability or accuracy.#COVID19 https://t.co/hHcXjimd4Q
— Dr Darren Saunders (@whereisdaz) April 29, 2020
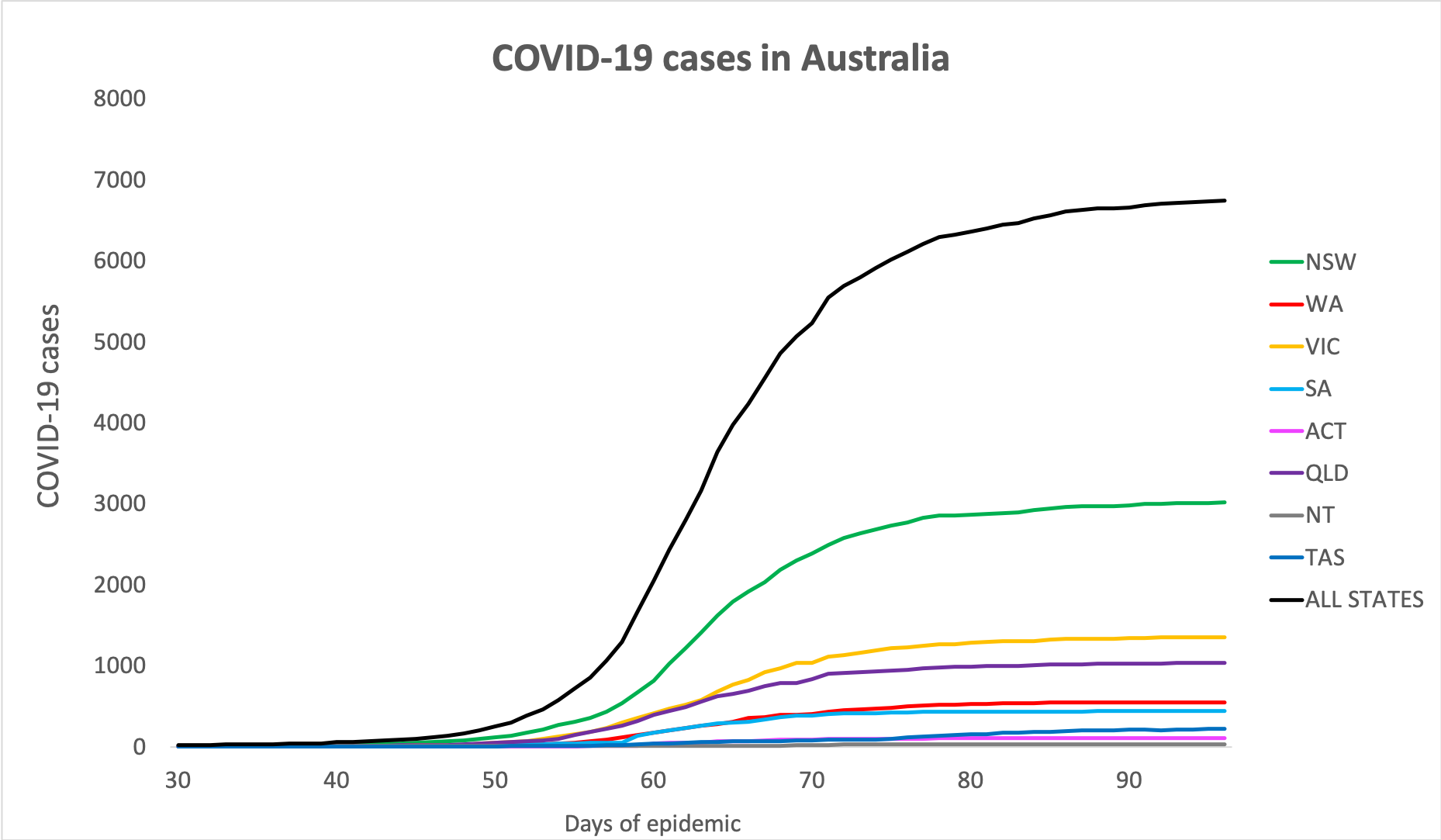
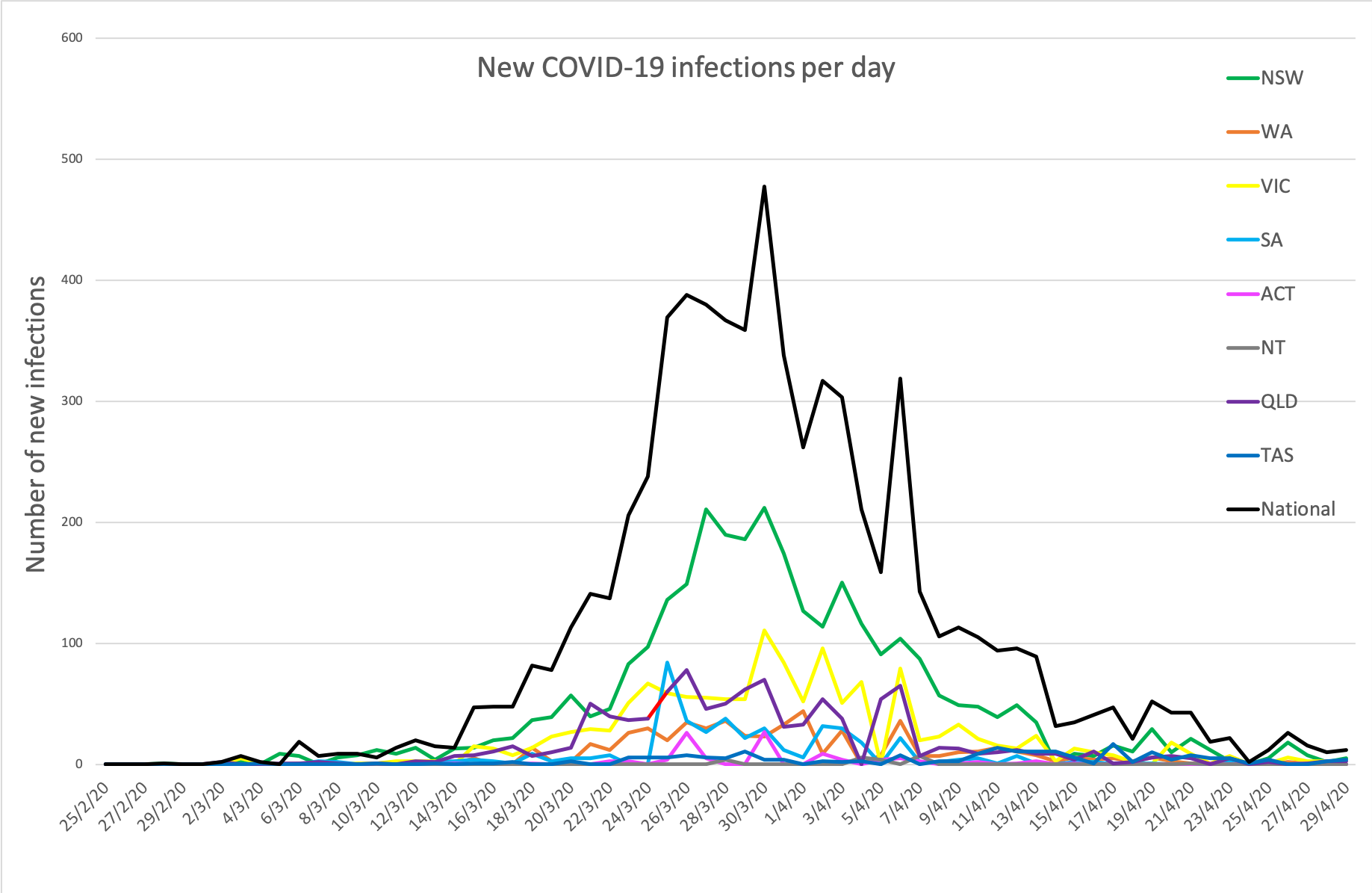
- Here are today’s confirmed COVID-19 infection rates from around Australia as of 6am this morning:
National – 6,746 (up 15) with 90 deaths and 5,685 listed as recovered
ACT – 106
NSW – 3016
NT – 27
QLD – 1034
SA – 438
TAS – 220
VIC – 1354
WA – 551
Disclaimer: The content on the Medical Republic COVID-19 blog is independently created by Medical Republic without input from Boehringer Ingelheim Pty Ltd. The views, information, or opinions expressed on the Medical Republic COVID-19 blog are Medical Republic’s own and do not necessarily represent those of Boehringer Ingelheim Pty Ltd. Boehringer Ingelheim Pty Ltd is not responsible for and does not verify the accuracy of any content on the Medical Republic COVID-19 blog.


