Specialists working in domestic violence have reported a significant increase in their caseload during the COVID-19 pandemic; both from an increase in new clients and an increase in the needs of existing clients.
Thank you for reading The Medical Republic‘s live COVID-19 blog.
Thanks to our sponsor and supporter for funding this project with an independent grant, Boehringer Ingelheim.
The latest
- Afternoon update: Specialists in domestic violence report an increase in demand during COVID-19.
- Morning update: kids on immunosuppressive therapy also only get mild COVID-19, clinical trial of IV immunoglobulin approved in US.
- Today’s updated COVID-19 infection figures for Australia
- Specialists working in domestic violence have reported a significant increase in their caseload during the COVID-19 pandemic; both from an increase in new clients and an increase in the needs of existing clients.
A survey of 53 specialists in NSW, by the Foundation for Alcohol Research & Education and Women’s Safety NSW, found nearly half reported increased demand for their services, and half also reported an increase in the role of alcohol use in domestic violence.
- Even children on long-term immunosuppressive therapy appear to have a mild course of disease with COVID-19 infection, according to a small cohort study published in The Lancet: Child & Adolescent Health.
The study involved 18 children from 16 paediatric nephrology centres across Europe, who were on immunosuppressive medication such as glucocorticoids, tacrolimus and mycophenolate for kidney disease and transplantation and who were diagnosed with COVID-19.
Most presented with fever or cough, only one child needed high-flow nasal cannula oxygen while more than 80% were fine without any oxygen support. Eleven were admitted to hospital but none ended up in intensive care. - The US Food and Drug Administration has given the go-ahead for a randomised, double-blind, placebo-controlled phase 3 clinical trial of intravenous human immunoglobulin to treat severe COVID-19. The product – Octagam – is already used to treat chronic immune thrombocytopenic purpura, but earlier case reports suggest it could help with COVID-19.
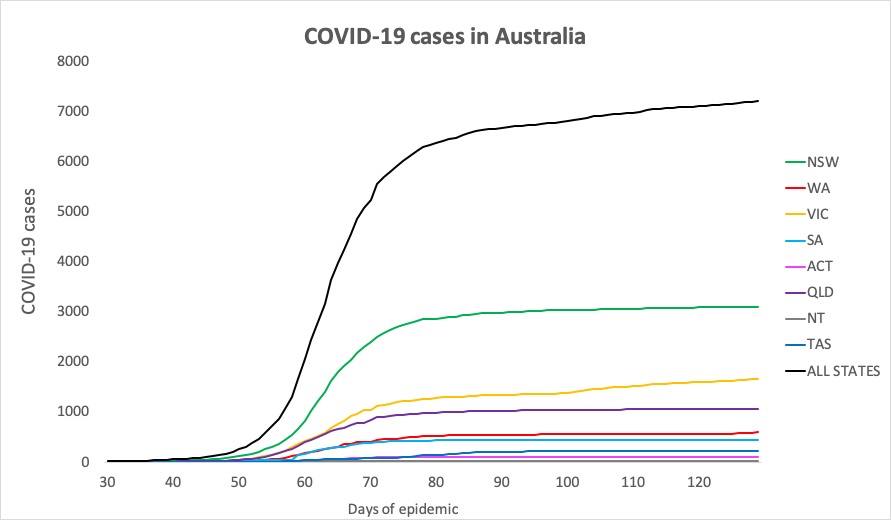
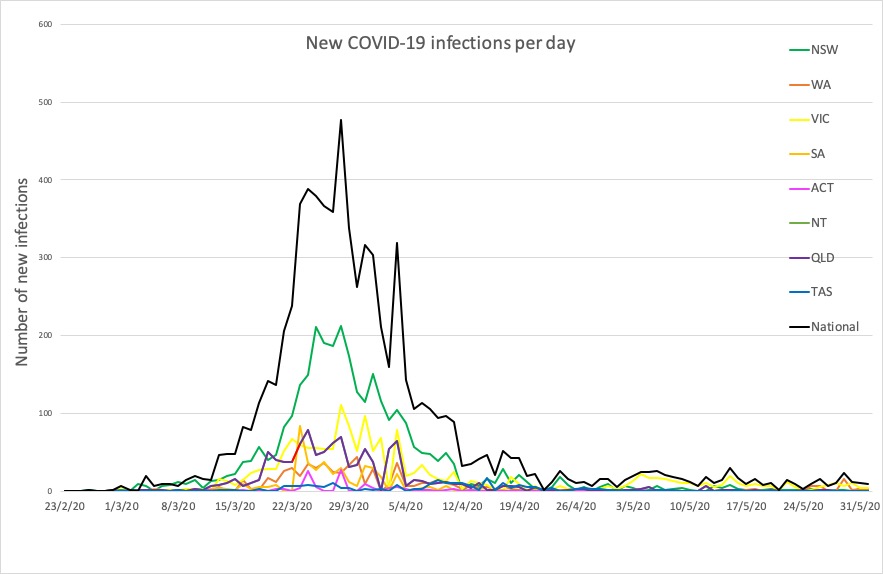
- No big jumps in infections in the 24 hours to 9pm yesterday, thankfully. Here are the latest confirmed COVID-19 infection rates from around Australia:
National – 7204, with 103 deaths and 6619 recovered
ACT – 107
NSW – 3098
NT – 29
QLD – 1058
SA – 440
TAS – 228
VIC – 1653
WA – 591
Disclaimer: The content on the Medical Republic COVID-19 blog is independently created by Medical Republic without input from Boehringer Ingelheim Pty Ltd. The views, information, or opinions expressed on the Medical Republic COVID-19 blog are Medical Republic’s own and do not necessarily represent those of Boehringer Ingelheim Pty Ltd. Boehringer Ingelheim Pty Ltd is not responsible for and does not verify the accuracy of any content on the Medical Republic COVID-19 blog.