The hydroxychloroquine plot thickens, with experts disputing the results of the hydroxychloroquine study published earlier this week in the Lancet.
That’s it for this week on The Medical Republic‘s live COVID-19 blog.
Thanks to our sponsor and supporter for funding this project with an independent grant, Boehringer Ingelheim.
The latest
- Afternoon update: hydroxychloroquine study results challenged, higher dose anticoagulants suggested for severe COVID-19, RCPA supports self-collection of COVID-19 samples.
- Morning update: Healthcare workers experiencing post-traumatic stress, TGA warns advertisers to toe the public health line, WHO publishes updated clinical guidelines on COVID-19.
- Today’s updated COVID-19 infection figures for Australia
- The hydroxychloroquine plot thickens, with experts disputing the results of the hydroxychloroquine study published earlier this week in the Lancet. The study suggested higher in-hospital mortality and new-onset arrhythmias in COVID-19 patients treated with hydroxychloroquine or chloroquine, either by themselves or in combination with a macrolide.
But in an open letter to the study’s authors and the editor of the Lancet, hundreds of researchers from around the world have criticised the study for failing to account for known and measured confounders such as disease severity and dose. They also pointed out there was no ethics review for the study – which used de-identified hospital data from a global collaboration – nor did the authors released the code or data used in the study.
The letter raised other concerns about errors in the data from Australia and Africa, about the high doses of hydroxychloroquine referenced in the study, and the statistical analysis. - The Royal College of Pathologists of Australasia has said it supports the use of self-collection of nasopharyngeal samples for COVID-19 testing.
Citing a recent trial which showed that self-collected specimens were an acceptable alternative to healthcare worker-collected specimens, the RCPA said in a statement that self-collection – with instruction from a medical professional – would improve the convenience and flexibility of COVID-19 testing and also reduce demands on PPE.
“Each time a new patient presents to a dedicated collection room, the collector is required to put on full PPE and then discard it once testing is complete,” said Dr Jenny Robson, Pathologist-in-Charge at Sullivan Nicolaides, in a statement.
The Public Health Laboratory Network has published guidance on self-collecting nasopharyngeal samples. - Adults with severe to critical COVID-19 can be treated with higher dose anticoagulants to help prevent blood clot-related events, according to the latest update from the National COVID-19 Clinical Evidence Taskforce.
The multi-organisational taskforce, which is working on living guidelines for the management of COVID-19, has advised that doctors consider increased prophylactic dosing of anticoagulants, preferably low molecular weight heparin, unless there is a contraindication. - Gilead has published the results of its dosing study of remdesivir, which was previously touted in a press release the same day as the NIH put out the results of its remdesivir trial.
This open-label, randomised study – published in the New England Journal of Medicine – was a comparison between five days and ten days of remdesivir, with no control group, in patients with severe COVID-19 but who were not ventilated.
The results suggested there were no significant differences in terms of clinical status between the shorter and longer courses of treatment.
- It’s a standard cliché of pharmaceutical advertising that a product will miraculously get you out of bed/pain/trouble and propel you into a brave and happy new world. Except in COVID-19, that sort of advertising could land you on the wrong side of the Therapeutic Goods Administration.
The TGA has warned advertisers that their campaigns – particularly coming into flu season – must remain consistent with current public health advice aimed at curtailing COVID-19.
“Promoting a product as enabling someone to attend a workplace, school or other activity outside of home while experiencing cold and flu symptoms (even if temporarily controlled) conflicts with the Department of Health’s advice to stay home when unwell,” they said in a statement. - The World Health Organization has published updated guidance for frontline healthcare professionals on caring for patients with COVID-19, from initial testing right through to hospital discharge. The guidance covers a large number of aspects of care, including management of conditions such as acute respiratory distress syndrome and pneumonia, managing pregnant women and infants with COVID-19, reporting of death from COVID-19 and even clinical research during the pandemic.
- Nearly half of frontline and second-line health care workers in Italy during the COVID-19 pandemic have reported symptoms of post-traumatic stress and a quarter have experienced symptoms of depression, according to a paper published in JAMA Network Open.
A survey of 1361 healthcare workers in Italy in the last week of March found GPs were more likely to report post-traumatic stress than other healthcare workers, as were younger and female healthcare workers.
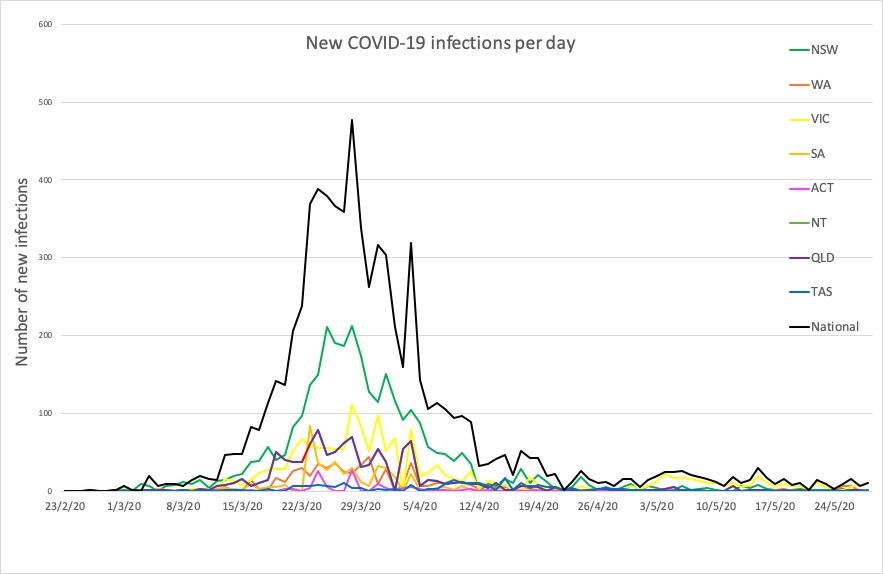
- Victoria has had another jump in cases – 10 in the past 24 hours – five of whom were all members of one household where there was a previous COVID-19 case. There were also three cases in returned travellers in hotel quarantine.
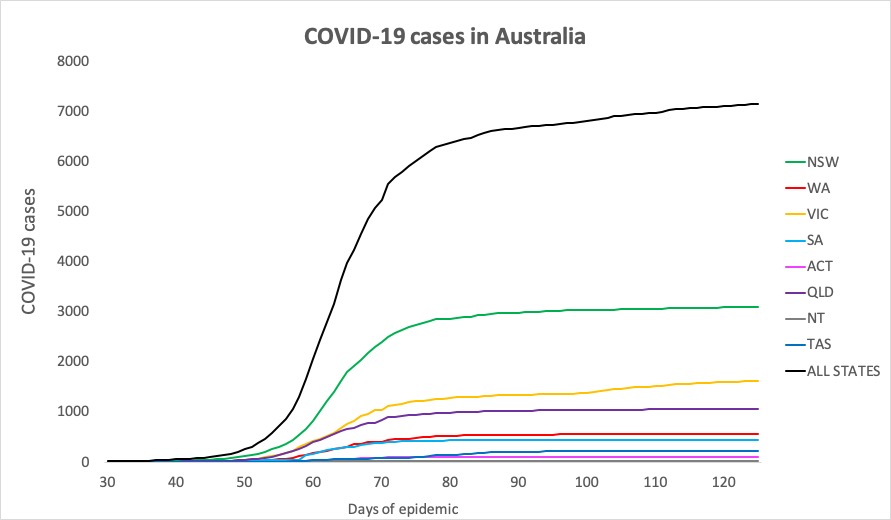
Here are the confirmed COVID-19 figures for around Australia, to 9pm yesterday:
National – 7150, with 103 deaths and 6580 recovered
ACT – 107
NSW – 3090
NT – 29
QLD – 1058
SA – 440
TAS – 228
VIC – 1628
WA – 570
Disclaimer: The content on the Medical Republic COVID-19 blog is independently created by Medical Republic without input from Boehringer Ingelheim Pty Ltd. The views, information, or opinions expressed on the Medical Republic COVID-19 blog are Medical Republic’s own and do not necessarily represent those of Boehringer Ingelheim Pty Ltd. Boehringer Ingelheim Pty Ltd is not responsible for and does not verify the accuracy of any content on the Medical Republic COVID-19 blog.


