The NIH-funded remdesivir trial shows the antiviral is associated with shorter time to improvement but only in patients not being ventilated.
That’s it for today on The Medical Republic‘s live COVID-19 blog.
Thanks to our sponsor and supporter for funding this project with an independent grant, Boehringer Ingelheim.
The latest
- NIH publishes its remdesivir trial data showing shorter hospitalisations compared to placebo.
- Another study shows higher mortality and arrhythmias with hydroxychloroquine.
- Today’s confirmed COVID-19 infection figures from around Australia.
- The National Institutes of Health has finally released the full results of its remdesivir trial in COVID-19, which it had previously announced by press release with much fanfare but little detail.
The double-blind, placebo-controlled trial, published in the New England Journal of Medicine, involved 1063 adults hospitalised with COVID-19 and lower respiratory tract ‘involvement’, who were randomised either to intravenous remdesivir or placebo for up to 10 days.
The primary outcome actually changed over the course of the study – something that was picked up by eagle-eyed observers– but ended up being time to hospital discharge or recovery.
On this count, the study’s preliminary results after early unblinding showed a significantly shorter median recovery time – 11 days for the remdesivir group compared to 15 days in the placebo group – but a non-significant trend towards reduced mortality.
One thing of note is that there seems to be a ‘sweet spot’ of disease severity where remdesivir is most effective. Patients who were ventilated or received ECMO showed no significant gains in terms of time to recovery, but those who needed supplemental oxygen but not ventilation showed the greatest improvements in recovery times. Those with less and more severe disease did not show statistically significant improvements.
“Our findings highlight the need to identify COVID-19 cases and start antiviral treatment before the pulmonary disease progresses to require mechanical ventilation,” the authors wrote.
Commenting on the change in the primary outcome, the authors said this happened because of changing understanding of the course of COVID-19 infection, and that it may have been more protected than originally anticipated.
And perhaps the most significant point that the authors made: “given high mortality despite the use of remdesivir, it is clear that treatment with an antiviral drug alone is not likely to be sufficient.”
- More bad news about hydroxychloroquine and chloroquine for the treatment of COVID-19; an international registry-based analysis of data from more than 96,000 patients has found they are associated with higher in-hospital mortality and an increased risk of ventricular arrhythmias.
A study published in The Lancet looked at data from the Surgical Outcomes Collaborative – a registry of de-identified electronic health records from 671 hospitals on six continents – to establish the effect of treatment with hydroxychloroquine or chloroquine, with or without a macrolide such as azithromycin. The primary outcomes were deaths in hospital from COVID-19, or the onset of new ventricular arrhythmias.
Overall, mortality was higher in all the treatment groups compared to the no-treatment control group. Patients taking hydroxychloroquine alone had a 33% higher risk of in-hospital mortality, those taking chloroquine alone had a 36% higher risk, those taking chloroquine with a macrolide had a 37% higher risk and those taking hydroxychloroquine with a macrolide had a 45% higher risk, compared to the control group.
The authors also found that all four treatment approaches were independently associated with an increased risk of new ventricular arrhythmia during hospitalisation. The increased hazard ranges from 2.3-fold greater with hydroxychloroquine alone to five-fold greater in patients taking hydroxychloroquine with a macrolide.
“Our large-scale, international, real-world analysis supports the absence of a clinical benefit of chloroquine and hydroxychloroquine and points to potential harm in hospitalised patients with COVID-19,” the authors wrote.
Despite this finding, a randomised controlled trial of hydroxychloroquine currently recruiting in the UK – the RECOVERY trial – has announced it will still go ahead. In a statement, the study organisers pointed out that the data so far suggesting net harm from hydroxychloroquine has largely come from two observational studies, but “randomised controlled trials are required to reach any conclusion about the benefit or harm of HCQ for COVID-19.”
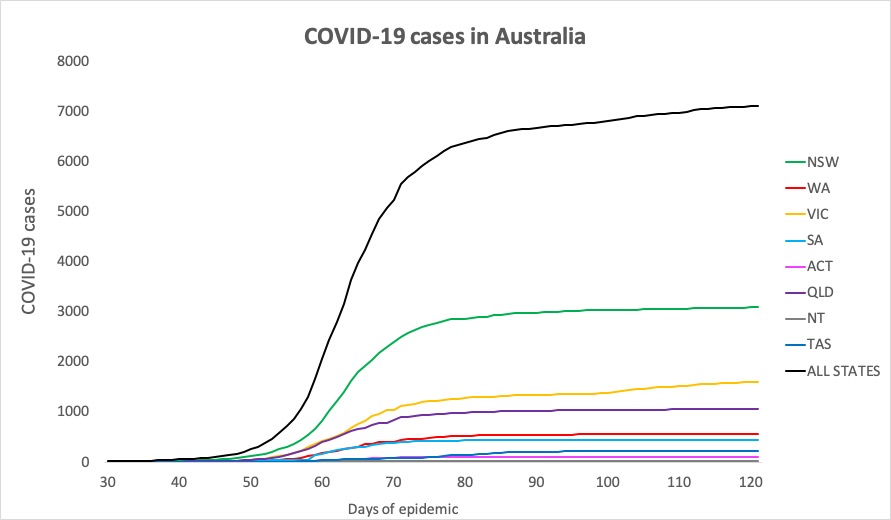
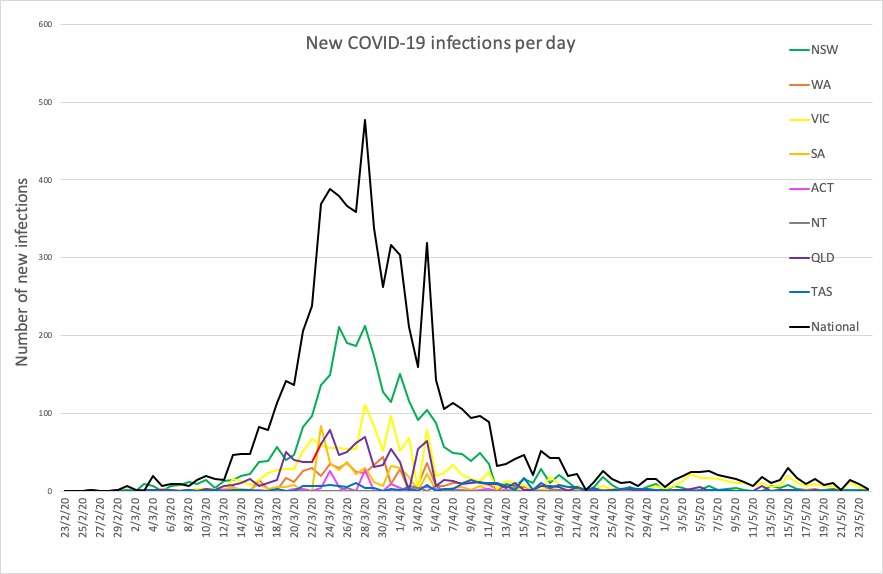
- Here are today’s confirmed COVID-19 infection figures from around Australia, to 9pm yesterday. And it’s still looking good, despite the lifting of lockdown measures.
National – 7109, with 102 deaths and 6506 recovered
ACT – 107
NSW – 3087
NT – 29
QLD – 1059
SA – 439
TAS – 228
VIC – 1603
WA – 557
Disclaimer: The content on the Medical Republic COVID-19 blog is independently created by Medical Republic without input from Boehringer Ingelheim Pty Ltd. The views, information, or opinions expressed on the Medical Republic COVID-19 blog are Medical Republic’s own and do not necessarily represent those of Boehringer Ingelheim Pty Ltd. Boehringer Ingelheim Pty Ltd is not responsible for and does not verify the accuracy of any content on the Medical Republic COVID-19 blog.



