The Australian Health Protection Principal Committee has given the nod to more opening up of elective surgery, suggesting that reaching stage 2 of the restrictions roadmap - whenever individual states decide to declare that stage - can trigger a return to up to 75% of normal surgical activity levels.
That’s it for this week on the The Medical Republic‘s live COVID-19 blog.
Thank you for joining us, and thanks to our sponsor and supporter for funding this project with an independent grant, Boehringer Ingelheim.
The latest
- AHPPC gives green light to acceleration of elective surgery resumption.
- Reduced expression of ACE2 gene in children’s noses may account for lower risk of COVID-19 infection.
- RCH Melbourne study finds low prevalence of COVID-19 in paediatric patients.
- The Australian Health Protection Principal Committee has given the nod to more opening up of elective surgery, suggesting that reaching stage 2 of the restrictions roadmap – whenever individual states decide to declare that stage – can trigger a return to up to 75% of normal surgical activity levels.
The conditional resumption of elective surgery was announced by the National Cabinet on Monday 27 April, and has seen a return to around 25% of previous elective surgery capacity.
Given the decrease in COVID-19 infections around the country, the unused hospital capacity in both private and public hospitals, and the firming up of PPE supply lines, AHPPC has advised it is now safe to increase the level of elective surgery activity.
However they stressed this should still be done in an “incremental and cautious” way to ensure there is still ICU capacity to cope with any localised outbreaks. They also advised that priority should be given to patients needing urgent surgery or who have already waited longer than expected for their procedure.
AHPPC also flagged that restrictions could still be reintroduced if there are further outbreaks of COVID-19.
- Could the reason that children appear less likely to get COVID-19 have something to do with relative lack of ACE2 receptors in their noses? It’s a theory that researchers in New York have put forward in a research letter in JAMA this week.
The ACE2 receptor plays a key role in COVID-19 infection, as this is the receptor that the SARS-CoV-2 uses to gain entry to human cells.
But a retrospective study of nasal epithelium samples from 305 individuals aged 4 to 60 years – who were participating in a study of nasal biomarkers for asthma – found that young children had the lowest levels of expression of the gene coding for the ACE2 receptor, and expression increased with increasing age.
The authors suggested this might explain the lower prevalence of COVID-19 in children. An accompanying editorial even raised the prospect that decreasing ACE2 expression in nasal epithelium might be a therapeutic option to reduce the likelihood of transmission of COVID-19. But it’s early days for this finding; more information may come from a recently-launched longitudinal study in 6000 children, looking specifically at risk factors for COVID-19.
2.45pm, 22 May
- Email subject line writing: when short isn’t necessarily sweet:

- A screening study of children presenting to the emergency department of Melbourne’s Royal Children’s Hospital for non-COVID-19-related issues has found less than 1% tested positive for SARS-CoV-2.
After the first confirmed case of COVID-19 at the hospital on the 21st March, every child presenting to the emergency department or the newly-established Respiratory Infection Clinic was tested for COVID-19, according to a paper published in Emergency Medicine Australasia.
Overall, 433 patients were tested until 19th April, and only 4 (0.9%) were found to be positive for SARS-CoV-2, none of whom were admitted to hospital or showed signs of severe disease. One was aged under 10 years, and the other three were over 10 years of age. One of the children had asthma but the others had no other comorbidities.
Three children had recently travelled overseas and all had had recent contact with a confirmed COVID-19 case. Three presented with headache, three had sore throat, and one had fever.
- Point-of-care serological testing for COVID-19 antibodies is a hot topic, with everyone wanting to know when it will happen and how reliable it will be compared to the existing RT-PCR testing.
The TGA has approved a number of COVID-19 point-of-care tests but there are restrictions on who can use them and they cannot be advertised to consumers. The tests are all currently being evaluated by the Peter Doherty Institute.
The concern is that the results of these tests could be misinterpreted. The TGA stresses that these tests don’t determine if an individual is infectious or has recently been infected. The tests look for two types of antibodies – IgM and IgG – which are produced by the immune system in response to infection with the SARS-CoV-2 virus, but don’t detect the virus itself.
“Accurate identification of a COVID-19 infection based on serology results requires an understanding of how the antibody response develops with time, and how there is a significant lag time between infection and the development of antibodies,” the TGA said in a statement. For example, it can take up to two weeks for these antibodies to appear following infection with SARS-CoV-2, so there is a risk of false negative results particularly if a patient is asymptomatic or has had symptoms for less than two weeks.
The TGA therefore recommends that point-of-care serology tests should not be used until at least two weeks after symptom onset.
The organisation also points out that serology testing is generally used to better understand the rate and pattern of infection in a population during an outbreak, which means it’s important for the results of these tests to be captured by public health reporting networks.
“If you are intending to offer point-of-care COVID-19 testing, staff in your facility should have the knowledge, experience and capabilities to safely and accurately carry out the specimen collection and testing, correctly interpret the results, provide current and correct health advice based on these results, and have robust record keeping and reporting systems in place,” it said.
- How Not To Share Your COVID-19 Test Results, by Donald Trump:
I tested very positively pic.twitter.com/lp4fE2bbai
— Sarah Cooper (@sarahcpr) May 21, 2020
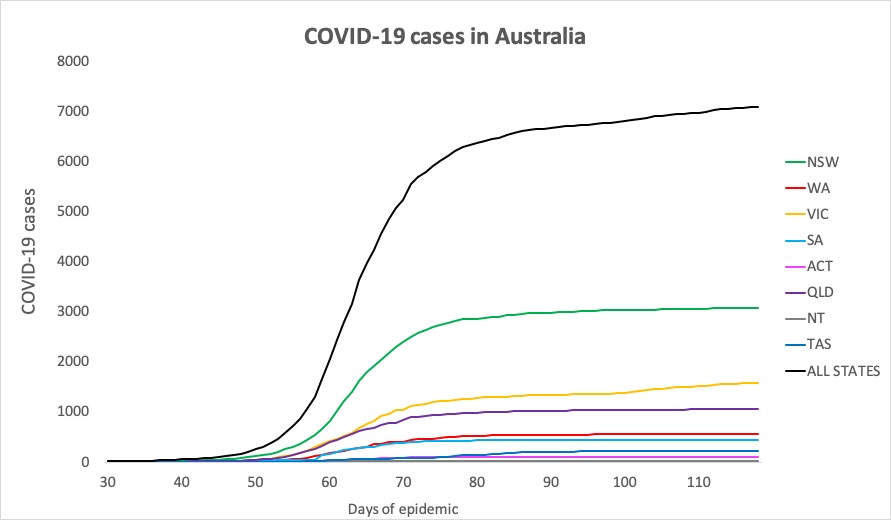
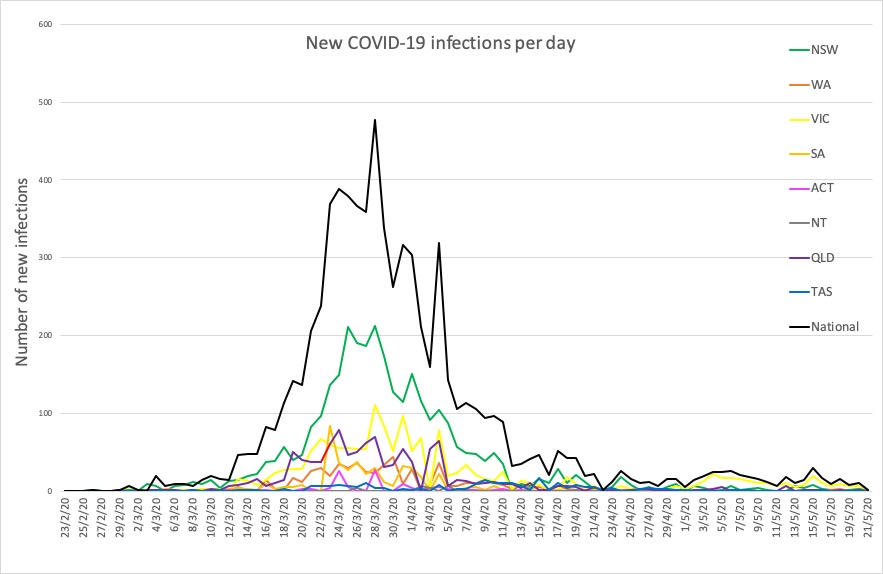
- Just two new cases of COVID-19 reported in Australia in the past 24 hours – one in Victoria and one in NSW. Here are the figures from around the country, to 9pm yesterday:
National – 7018, with 100 deaths and 6472 recovered
ACT – 107
NSW – 3082
NT – 29
QLD – 1058
SA – 439
TAS – 228
VIC – 1581
WA – 557
Disclaimer: The content on the Medical Republic COVID-19 blog is independently created by Medical Republic without input from Boehringer Ingelheim Pty Ltd. The views, information, or opinions expressed on the Medical Republic COVID-19 blog are Medical Republic’s own and do not necessarily represent those of Boehringer Ingelheim Pty Ltd. Boehringer Ingelheim Pty Ltd is not responsible for and does not verify the accuracy of any content on the Medical Republic COVID-19 blog.



