The clinical trial has been put on hold for what researchers say is a routine investigation of a possible serious adverse event.
Welcome to The Medical Republic‘s COVID Catch-Up.
It’s the day’s COVID-19 news in one convenient post. Email bianca@biancanogrady.com with any tips, comments or feedback.
9 September
- AstraZeneca has halted clinical trials of the Oxford vaccine to investigate a possible serious adverse event.
- Nearly 800 cases of COVID-related multisystem inflammatory syndrome in children reported in US.
- Sewage testing raises warning flag of SARS-CoV-2 in Victorian community with no active cases.
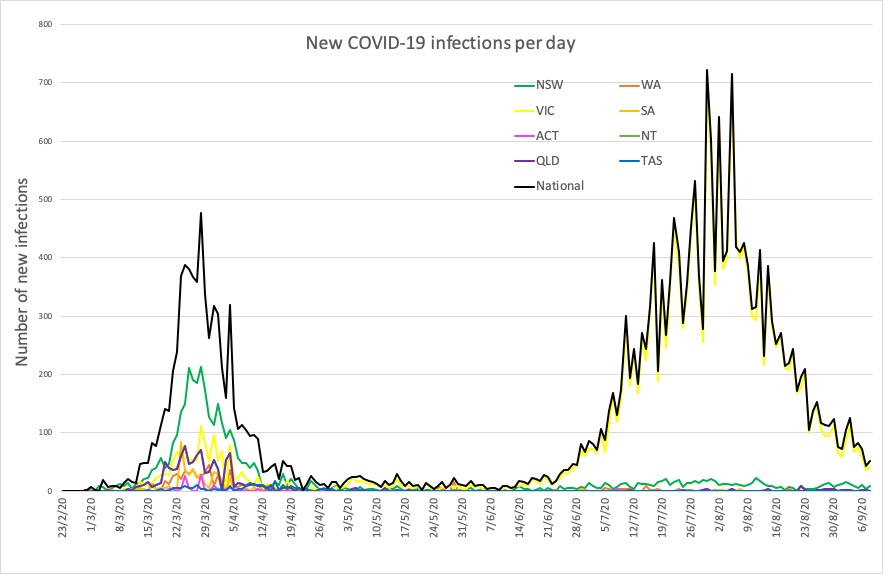
- Latest COVID-19 infection numbers from around Australia.
- The clinical trial of the Oxford vaccine has been put on hold as researchers investigate a possible serious adverse event in one of the participants, according to medical news outlet STAT.
The site reported that vaccine manufacturer AstraZeneca had initiated the hold as a standard process to allow review of the safety data from the trial, but at this stage there was no further information about the event. A spokesperson for the company told STAT that this was a routine procedure whenever there was potentially unexplained illness in one of the trials. - Nearly 800 cases of COVID-19-associated multisystem inflammatory syndrome in children and 16 deaths have now been reported to the US Centers for Disease Control since the organisation started tracking this condition in early May.
Describing it as a rare but serious condition associated with COVID-19, the CDC reported that most cases developed within 2-4 weeks after infection with SARS-CoV-2, and 99% of cases tested positive for the virus. The remaining 1% had been in contact with someone with COVID-19.
The average age of cases is 8 years, and most cases are seen between the ages of 1-14 years, with the highest number in those aged 5-9 years. More than 70% of cases have occurred in children of Hispanic or Black ethnicity, and just over half were male.
Meanwhile, a systematic review of 39 observational studies of multisystem inflammatory syndrome in children, published in EClinicalMedicine, found all patients with the condition presented with fever, nearly three-quarter had abdominal pain or diarrhoea, two-thirds had vomiting, and around half also presented with conjunctivitis or rash.
Patients showed abnormal levels of inflammatory markers such as C-reactive protein, cardiac markers, and coagulative markers, and around half of them had abnormal echocardiograph findings such as depressed ejection fraction.
Seventy-one percent of children diagnosed with the condition were admitted to intensive care, and just over 22% required mechanical ventilation. - Sewage testing across Victoria has picked up fragments of SARS-CoV-2 RNA in wastewater from Apollo Bay where there are currently no known active cases of COVID-19.
The discovery has led to an increase in testing in the area, and locals with even mild symptoms have been urged to get tested and self-isolate until they get their results.
The Victorian Health department said the finding could be the result of an undetected case in the community, but could also be caused by persistent viral shedding from someone who had previously had COVID-19.
“It can take several weeks for someone to stop shedding the virus and further analysis is required to assess the significance of the preliminary result,” the department said in a statement. - Here are today’s confirmed COVID-19 infection numbers from around Australia, to 9pm Tuesday:
National – 26,374, with 770 deaths
ACT – 113 (0)
NSW – 4126 (9)
NT – 33 (0)
QLD – 1134 (1)
SA – 465 (1)
TAS – 230 (0)
VIC – 19,615 (55)
WA – 658 (1)