With autopsy rates falling sharply, the work of the Sydney Brain Bank is becoming increasingly vital
“Nowadays the only thing we take brain biopsies for is if you’ve got an absolutely awful disease or a brain tumour, because you’ve got to have a really good reason to open up the brain cavity and take tissue out.”
Dr Claire Shepherd explains the societal shift away from post-mortems while washing her hands over a stainless-steel sink outside the labs in the Sydney Brain Bank, where she oversees one of Australia’s biggest collection of brains and spinal cords.
Her work is increasingly vital, as Australia and the rest of the world witness post-mortem rates plummeting to record lows, robbing the community of critical chances to better understand the diseases with which we live, and from which we die.
Falling rates of autopsies have been blamed on broad factors, such as the cost and community reluctance, to the fear of medicolegal consequences for the doctors who order them.
But another major cause may be that medical research is a victim of its own success. Imaging and blood tests are improving rapidly, giving us a wealth of information about health problems we face during life, but they are incomplete.
One review of almost 6000 autopsies of intensive-care unit patients found that 28% had at least one misdiagnosis, and 8% had a potentially lethal misdiagnosis.
Similar figures have been seen in other post-mortem studies, validating the concern that there remains a gap between what we think we know in life and the information gleaned after death.
When it comes to neurodegenerative diseases, such as Alzheimer’s disease, where the definitive diagnosis is not made until after death, the autopsy is even more critical, Dr Shepherd explains.
“It’s only until we get the tissue here that we can get the definitive diagnosis,” she says. “Otherwise, we lose a lot of information when a patient dies.”
Researchers are working hard to develop reliable biomarkers of disease and identify the relationship between clinical disease and underlying pathology. But until imaging tools can achieve the gold standard that post-mortems can, clinical trials could be seriously flawed, according to Dr Shepherd.
“If you have got a group of cases you say are Alzheimer’s disease, but in fact 20% of them turn out to be something else, you can never, ever, hope to get success in your clinical trial for a treatment, because you’ve already started up with a faulty experimental design.”
Still, closing mortuaries, falling autopsy rates and the shrinking pool of qualified neuropathologists highlights our society’s shifting priorities.
“A lot of countries have cut down. I guess they put the money into the sick instead of the dead.”
It is unlikely we’ll be able to turn back the clock and make autopsy widespread again, so the future of post-mortem analysis looks set to rest in the research setting.
The Sydney Brain Bank, which operates out of the Neuroscience Research Australia (NeuRA) institute, has amassed approximately 550 brains and 80 spinal cords since its launch in 2009.
By 2016, approximately 13,000 tissue samples had been supplied to researchers in countries such as Finland, South Korea, Sweden, the UK and the US. More than 200 presentations and publications sprung from research done on the brain and spinal tissue sourced there.
There are a handful of brain banks in Australia, each with a slightly different focus and different operations. Sydney Brain Bank is primarily interested in research around neurodegenerative and ageing disorders.
Instead of relying on coroner’s courts or individual doctors to request autopsies, the Sydney Brain Bank works with nine separate donor programs through which volunteers can sign up to donate their brain after death. This includes donor programs for patients with motor neuron disease, Alzheimer’s and frontotemporal dementia, movement disorders and ageing.
As well as caring for participants in life, these programs enable the researchers to amass a rich set of clinical data about the donors while they are alive.
Researchers have access to longitudinal information that includes brain imaging, blood tests for biomarkers and neurophysiological information.
There is, thankfully, no shortage of people happy to donate their brains for research, according to Dr Shepherd.
The brain bank shares the big, modern NeuRA building with groups studying everything from traffic accidents to sleep. This is useful in recruiting volunteers, many of whom come from other research programs in the institute.
For example, an individual may decide they want to take part in a study on falls and balance run by another team in the building, and in doing so be asked if they want to become a brain donor as well.
Dr Shepherd and her team then get the benefit of knowing that the donor participated in the falls and balance study, which provides them with specific information on the kinds of falls they had during life.
One example of this collaboration is a study Dr Shepherd is undertaking with Professor Stephen Lord, who oversees the falls and balance research, into the neuropathology of concussion.
“We know that repeated or severe head injuries are related to an increased incidence of neurological or psychiatric disorders,” Dr Shepherd says.
“So I’m doing a study in a group of people who fall a lot, who are potentially at risk of head injury, to see whether they have any differences of pathology.”
While these individuals don’t often have overt neurodegenerative disorders, they do appear to have subtle pathological changes in line with what we know as chronic traumatic encephalopathy, she says.
But there is still more work to be done in understanding how and why some people develop complications from head injury and others don’t.
Unlike many other tissue banks, the Sydney Brain Bank is involved in the collection stage as well as the characterisation, storage and distribution of the brain and spinal tissue.
When it comes to collection, time is of the essence. A staff member is rostered on to collect the brain or tissue from the mortuary, open every Monday to Saturday from 9am to 5pm, including Christmas and New Year’s Day, to ensure that it is process as soon as possible and the tissue quality is preserved.
Research associate Andrew Affleck oversees brain and spinal cord removal, which takes between half an hour and an hour for the brain and potentially another two hours for the spinal cord.
For his PhD, Mr Affleck is investigating whether anti-hypertensive drugs have a protective effect against Alzheimer’s disease, and if early findings are borne out, to determine which specific drugs or classes may potentially be used to prevent dementia.
For Dr Shepherd, research into human brain tissue will be “absolutely crucial” to advancing our understanding of these disorders. “Even if we can delay the onset of these disorders, rather than prevent them altogether, it will have a significant impact.”
Dr Shepherd recalls the final years of her grandmother’s life, reflecting that if her dementia had been delayed five years and she had instead died of a heart attack, her grandmother would have been spared those last years of suffering and struggling in a nursing home.
Collection and diagnosis
Each year, around 50 more donors’ brains are added to the collection at the Sydney Brain Bank.
But it’s a far cry from the days of neurologists calling up with a fascinating case that he or she wanted a diagnosis on.
Providing a diagnosis to clinicians and families is certainly still an important facet of the work that Sydney Brain Bank does.
The thorough examination can provide some startling results, with the brain bank giving one example of a man who was treated for years for Parkinson’s disease, only for the doctors and loved ones to find out that he in fact suffered from frontotemporal dementia and Alzheimer’s disease.
The genomic investigations done on donors can also reveal important information on heritable diseases relevant to family members. But it makes more sense for the brain bank to allocate their limited resources to doing this for patients who have already been recruited and tracked as part of the donor programs.
When each brain and spinal cord takes an average of four or five months to characterise, and an average of $8000 to collect, characterise and store, the priority is for cases that can get the best possible research outcomes.
Part of that is to group similar cases together, almost like a cohort, for researchers. Those weird and wonderful cases? They are an “n of one”, Dr Shepherd says, and have fewer research teams interested and hence less research output.
There is a healthy donor program, but having healthy, age-matched controls is also critical. Unfortunately, patients with neurodegenerative diseases such as Parkinson’s and Alzheimer’s tend to die younger, perhaps around age 70, whereas a healthy individual who dies of a heart attack or cancer will very often live until the age 80 or 90 years.
Regardless, during post-mortem both healthy and diseases brains undergo a thorough screen for pathologies of proteins known to be involved in ageing
“It might seem like an onerous task, but actually there are only a handful of proteins used to characterise these age-related neurodegenerative disorders,” Dr Shepherd explains.
But it is not simply a case of looking down the microscope and saying ‘Oh, it’s got that protein, then it must be this’, Dr Shepherd tells The Medical Republic.
In different disorders the same protein is expressed in different cell types and in different regions.

Autopsy and dissection
To ensure that the tissue donation is utilised fully, half of the brain is frozen and the other half fixed in formalin.
While it may seem macabre, the dissection isn’t a gruesome process, Dr Shepherd says genially.
Like her operation, Dr Shepherd is precise. Before starting one interview she apologised earnestly for being late, at three minutes past the agreed time.
With a faint English accent and carefully chosen sentences, Dr Shepherd explains how the first step is an incision made in the scalp, where it can be replaced and sewn up in case the donor’s family wants an open casket.
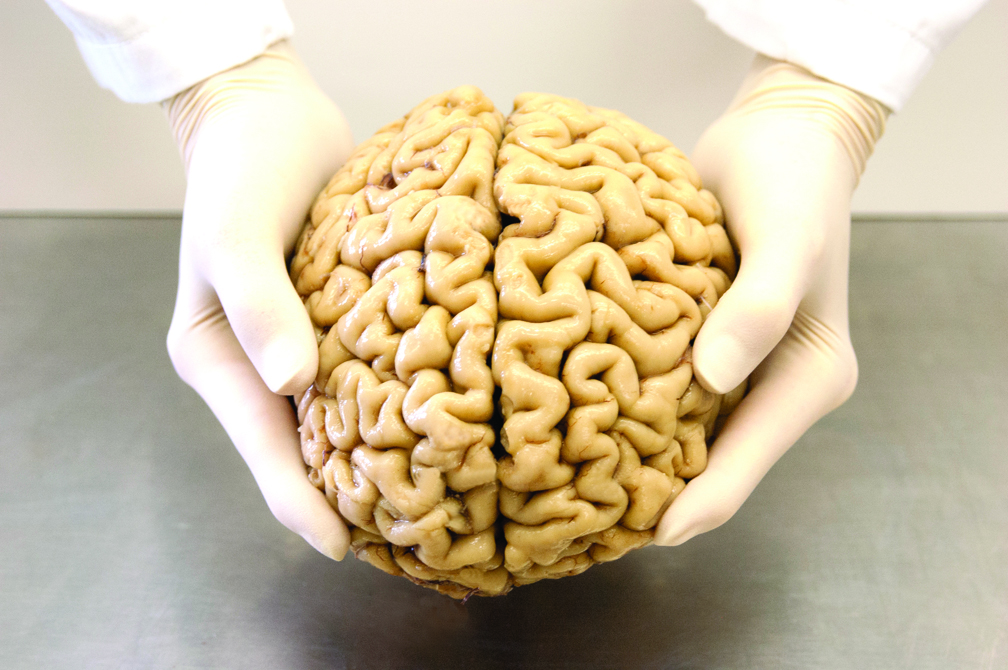
Pulling out a lidded bucket with an identification number on a label, Dr Shepherd explains that the first step of the characterisation process is an examination of the gross external features. Each surface of the brain gets photographed, and the pictures archived so that they can re-examine a brain if, later, there are questions of an abnormality being missed.
“We examine the external features, take brain weights, measure the length of the brain, make standard assessments of the brain and note if there are any abnormalities,” she explains as she walks over to a big, silver workspace that looks more than a little like a bain-marie.
She puts the bucket on the countertop and opens the lid, revealing the brain inside: 1.3 kilograms of grey, rubbery-looking ridges and furrows, with the translucent, thin sac of meninges and blood vessels still clinging to the outside.
Dr Shepherd, Mr Affleck or another staff member will then cut the brain in half, down through the twisted folds of the cortex, the home of consciousness, then into the older architecture of the brain.
Mr Affleck’s knife slices through the thick bundle of nerve fibres connecting the brain’s two hemispheres, the corpus callosum, and then through the even more ancient structures such as the thalamus, amygdala and cerebellum.
The next step is the dissection, where Mr Affleck cuts half-centimetre slices to examine the internal features to see if there are any abnormalities like evidence of stroke.
“It is like an egg,” Dr Shepherd explains. “It can look fine on the outside but be bad on the inside.”
The half of the brain that seems most affected gets fixed in formalin so that it develops a robust consistency that preserves cell morphology and makes it easy to cut and stain with antibodies. The other gets put in a freezer set to minus 80 degrees.
But first, Mr Affleck takes standardised blocks of tissue of commonly requested regions from this half, such as the hippocampus, the entorhinal cortex, and regions of the brain stem such as the substantia nigra. These are frozen separately so that the tissue isn’t damaged by repeatedly thawing and refreezing every time a research team requests a sample of the area.
Different brain banks have different methods to quickly freeze the tissue to preserve its integrity, some using liquid nitrogen, others use ice-cold isopentane, and others still put their tissue on copper plates.
A donor’s brain and spinal tissue may stay frozen in those archives for at least 15 years, unless the tissue is completely used. A valuable control brain could even be used in as many as 50 different studies before all the regions of interest are exhausted.
A stainless-steel deli-meat slicer sits in the corner of the room, which the researchers use to slice the brain tissue into consistent widths.
The lab is sprinkled with kitchen utensils, from the kitchen sponges by the sink to the modern microwave. Except, instead of colourful food in tupperware containers, there are powders, and instead of sauces, there are mysterious-looking liquids.
Keeping half the brain frozen means that researchers can use the tissue to study genomics and proteomics. The fixed brain, on the other hand, is for examination under the microscope. This is where Dr Shepherd and her team analyse the cellular structure, looking at changes to cells and deposition of the abnormal proteins that characterise many brain disorders.
Not all other brain banks collect spinal cords, but the process is similar, with some regions collected for freezing and others for fixing. It may be time-consuming, but Dr Shepherd says recent research suggests the spinal cord plays a larger role in neurological disorders.
“We’ve always known disorders like motor neuron disease have a strong spinal cord involvement, but we’re starting to understand that Parkinson’s disease also shows pathological changes in this region,” she says.
Studying tissue
Once the brains and spinal cords have been collected and characterised, researchers are able to submit an application for specimens.
Each year, 30 to 40 tissue request applications will make it through the rigorous screening process, then be evaluated by an independent review panel before being approved. Applications go to NSW Brain Banks, which incorporates the NSW Brain Tissue Resource Centre at the University of Sydney, a brain bank specialising in alcohol abuse disorders, schizophrenia and healthy control tissue.
The job of the Sydney Brain Bank isn’t over after collection. They might help a researcher design their study, recommending more appropriate areas of the brain to study based on what is available or directing them to other research groups working on similar projects.
Some research groups need help finessing their study for human tissue. Although scientists have created animal models of many neurodegenerative disorders, they don’t fully recapitulate all the features of the human disease process, Dr Shepherd says.
For example, most of the Alzheimer’s disease cases at the brain bank have co-existing pathology. But when seeking tissue samples, some researchers have initially requested pure Alzheimer’s disease cases for their studies because they don’t want to ‘muddy the waters’.
But this is the reality of Alzheimer’s disease, Dr Shepherd explains, “and if we’re going to treat it, then we have to treat the entirety of it”.
Those who do use tissue have to report back on their study outcomes and whether there is any leftover tissue to be returned.
“We are the custodians of the tissue samples, and I need to make sure that they are used for world-class research.”


