The key to elective adjuncts is tailoring them for individuals who are already engaged in active therapies
Beyond education, weight loss and exercise, which should be prescribed to all patients with OA knee pain, other treatments are optional, but likely to be of value if they can promote useful exercise.
These treatments can be tailored to the individual depending on the severity of the symptoms, and perhaps the “phenotype” of their knee OA. They have a role as elective adjuncts, tailored to specific individuals who are already engaged in active therapies, including exercise and diet therapy. These include: pharmaceuticals such as NSAIDs, nutraceuticals like glucosamine-chondroitin, orthotic devices, electrotherapeutic modalities, acupuncture, injectable treatments, and even stem-cell injections.
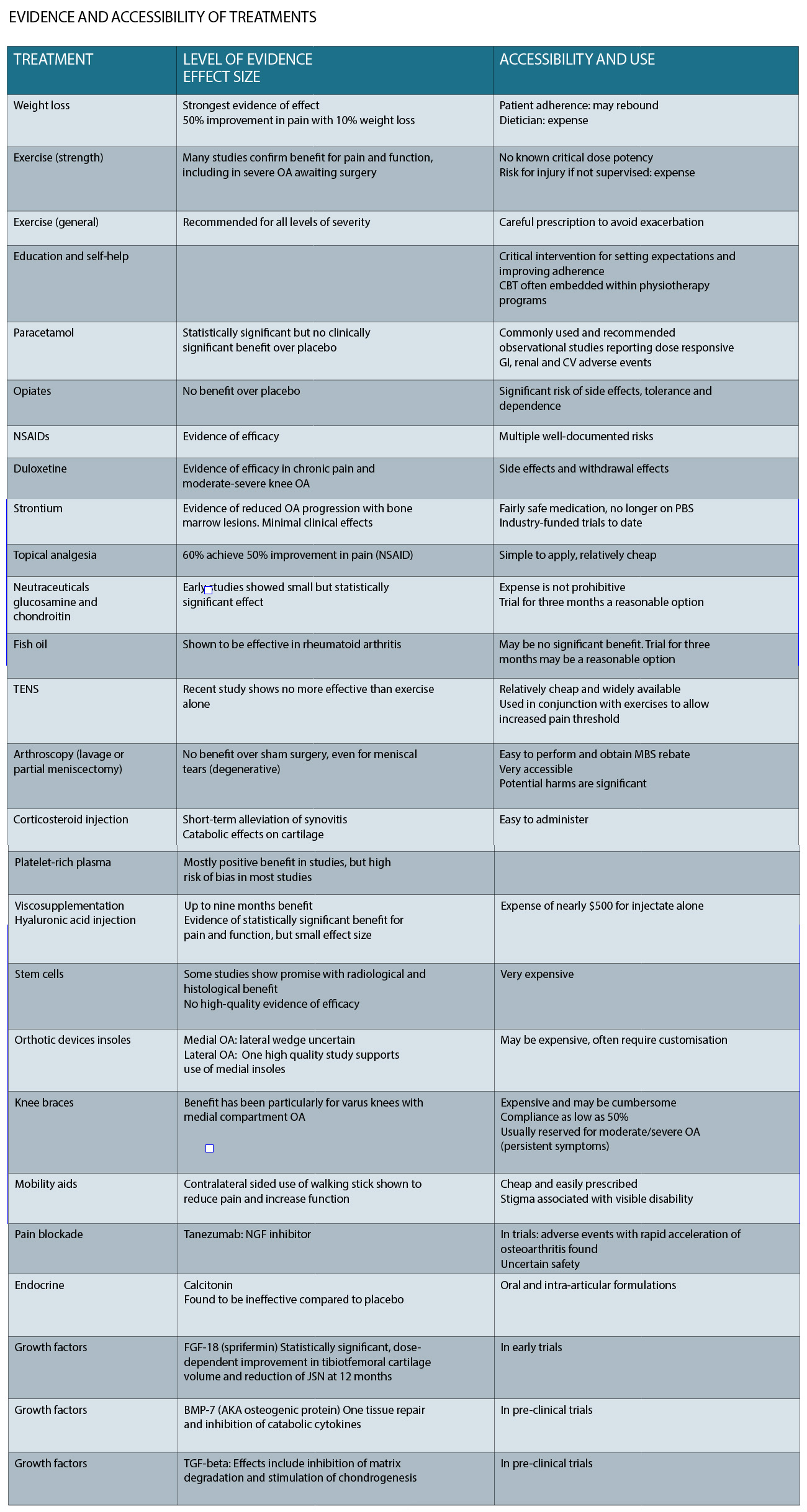
A table summarising the level of evidence and accessibility of each of these treatments is presented below.
It must be acknowledged that treatment effects in studies and clinical practice cannot be entirely attributed to the action of an intervention, especially for pain scores which are subjective. Zhang (2008) shows very elegantly that the effect size of the placebo effect in studies of OA is substantial compared with control groups with no active treatment. The size of the placebo effect increases significantly with increasing invasiveness of treatment (injections or even surgery).
Analgesics, including paracetamol, topical and oral non-steroidal anti-inflammatory drugs (NSAIDs) and duloxetine have evidence of effectiveness.
Paracetamol is commonly used at up to 4g a day in formulations that include the 1.33g formulation called “Panadol Osteo”. Systematic review evidence shows that the effect size for paracetamol, although statistically significant, would be too small to be clinically meaningful. Systemic side effects (including GI ulceration and bleeding) become significant at doses above 3g/day, and are increased when paracetamol is combined with NSAIDs.
NSAIDs are effective for improving pain and function in OA.
The advantage of topical formulations for joints with superficial joint margins (such as the knee or hand joints) is reduced systemic side effects. Sixty percent of patients obtain a 50% pain reduction using this simple method. A similar topical agent that aims to reduce local nociception is topical capsaicin, which improves pain in 80% of patients, and has negligible toxicity. On a practical level, patients are advised to use these treatments with a plastic cover (e.g. Gladwrap) overnight to allow the gel to penetrate the skin. Simply rubbing them in may not allow enough contact time.
Oral NSAIDs are effective and have a moderate effect size. The most consistent NSAIDs meeting minimal clinically significant effectiveness have been measured with diclofenac total 150mg/day and etoricoxib 60mg/day. Cox selectivity has the theoretical advantage preserving the platelet and mucosal functions of Cox, but does not eliminate GI adverse events, and increases cardiovascular events. Combination with a proton pump inhibitor is suggested for patients requiring longer-term use beyond two weeks and those with risk factors for GI side effects. In contrast, opiates have limited evidence of effectiveness and many intolerable side effects, including dependence.
Colchicine is an agent that inhibits the innate immune response via neutrophil chemotaxis, and also reduces elastase activity. Particularly in patients with an “inflammatory” phenotype, colchicine added to regular NSAIDs appears to be significantly more effective for pain and function. The main side effects are gastrointestinal upset and diarrhoea.
Duloxetine, an SNRI antidepressant, has shown efficacy in the treatment of pain associated with OA and other conditions such as low back pain. The effect size is at least moderate for both pain and function, based on a recent meta-analysis. It is generally taken at night time, with a starting dose of 30mg for a week, increasing to 60mg as tolerated. There is stigma associated with the use of antidepressant agents, which, along with psychotropic side effects, may limit its usefulness. In patients with coexisting depression, there is a documented black-label warning for increased risk of suicide.
There are physical treatments that have been used with the goal of achieving a short-term “pain-gate” analgesic effect. Acupuncture (dry-needling) is thought to work via this mechanism. Transcutaneous electrical nerve stimulation (TENS), is a similar approach, and machines are quite affordable now. Clinical evidence for both of these is very weak. With their low cost and low risk of adverse effects, patients may opt to trial these therapies.
Orthotic supports may be useful in patients who have evidence of abnormal joint alignment. They have to potential to facilitate increased physical activity and its benefits, via reducing mechanical loading on an affected knee compartment However, these devices require some customisation and fitting, are expensive and knee braces, in particular, are associated with limited adherence as they can be cumbersome. Don’t forget about simple walking sticks for more limiting knee OA, which definitely works for pain and functional improvement, and can easily be purchased at most pharmacies.
Nutraceuticals have gained popularity in patients with osteoarthritis, including glucosamine sulfate and chondroitin sulfate (GS/CS), fish oil and avocado-soybean unsaponafiables (ASUs). There is very limited evidence of effectiveness of any of these agents when placebo controlled trials are conducted. One study showed reduced loss of joint space with glucosamine chondroitin sulfate over two to three years; but no change in pain or functional scores.
A recent high quality RCT showed that six months of treatment with GS/CS, yielded no improvement over placebo for symptomatic knee OA. Fish oil studies have confused the picture recently, with the low-dose control group (4g canola oil and 0.5g fish oil) outperforming the treatment group (4.5g fish oil) in a recent RCT. Given their low toxicity or harm, a commonsense approach is to give patients the freedom to trial an agent for three months, and reassess standardised pain and function scores to assess the effect.
Injectable adjuncts for knee OA mainly centre around glucocorticoids, hyaluronans, platelet-rich plasma and more recently, autologous conditioned serum. Whether these agents work via their proposed mechanism, or whether other factors are at play such as placebo or symptomatic improvement from mandated rest that occurs following injection, remains uncertain. All joint injections carry a small (less than 1 in 10,000) risk of septic joint or haemarthrosis. Nonetheless, they do have a place in facilitating active therapies in OA management.
Glucocorticoid or “cortisone” is just a synthetic analogue of cortisol, and has a slow onset of effect in dampening cellular transcription of inflammatory mediators. It provides short-term symptomatic relief, for an average of one week up to four weeks. Studies have shown that regular cortisone injections increase the rate of articular cartilage loss over two years. However, in the very synovitic and inflamed knee they have a role in reducing pain and limiting accelerated joint destruction associated with this inflammation. Think of cortisone as a rescue therapy for patients who have severe symptoms.
Hyaluronans (HAs), are synthetic glycosaminoglycans, produced from rooster’s comb, which can theoretically help restore synovial fluid viscosity in osteoarthritic joints, and influence joint inflammation. Large scale (mostly industry-funded) trials show a small but statistically significant effect compared with placebo or corticosteroid. Early studies suggested a trajectory of effect with onset at four weeks, peak effect at eight weeks, and duration of effect until around nine months. When industry-funded trials were removed from one meta-analysis, the superiority to placebo disappeared.
The most common adverse event is a post-injection flare, which can be very dramatic and mimic a septic joint. The products themselves cost a little under $500. Think of hyaluronans as a “grease and oil” change for patients.
Platelet-rich plasma is an autologous blood product that aims to isolate and use platelets and their growth factors to influence the catabolic and inflammatory processes in the OA joint. There are many different methods and only recently has a classification system been developed that separates platelet-rich plasma based on the concentration of platelets and leukocytes (rich or poor).
Studies show promise for platelet-rich plasma, but are almost universally at high risk of bias and have used hyaluronans as the control injection. A recent meta-analysis suggested that leukocyte- poor platelet-rich plasma was most effective for knee OA.
Adverse events are rare, and there is inherent antibacterial leukocyte activity in PRP that may limit the risk of joint infections. Costs can vary between about $300 and $600 per injection.
Think of platelet-rich plasma as a growth factor transplant, that is probably more useful for younger patients.
Autologous conditioning serum (e.g. orthokine) is another autologous blood product that concentrates Interleukin-1 receptor antagonists (IL-1Ra). IL-1RA is thought to be a central mediator to cartilage loss.
One RCT demonstrates superiority over HA and saline, with a large effect size on pain and function It usually costs around $1000 for the course of treatment.
Think of autologus conditioing serum as an attempt to combine the actions of cortisone and platelet-rich plasma.
Recently, mesenchymal stem cells (MSCs) obtained from blood, fat or bone marrow have been used in clinical practice. Similar to the evidence for platelet-rich plasma injections, there are promising results but high risk of bias among all of the RCTs to date.
Stem-cell injections appear to pose little risk of physical harm with no major adverse effects reported. A recent systematic review reveals the high risk of bias of all RCTs that have been performed, and recommended against clinical use of mensenchymal stem-cell injections.
The Australasian College of Sport and Exercise Physicians position is that stem cell therapy should not yet be offered as a part of routine clinical practice, but should continue in research settings until safety and efficacy are demonstrated.
This treatment costs around $10,000 for the harvesting, processing and re-injection of stem cells. Currently, stem cells might be a justifiable last resort for joints that don’t have better surgical options (like ankle joints).
WHEN IS IT TIME FOR SURGERY?
In the absence of mechanical symptoms and a trial of rehabilitation, there is no role for knee arthroscopy in OA knees. Well conducted RCTs have consistently shown that the outcomes for surgery are no better than physiotherapy alone, with the caveat that some patients do fail physiotherapy and cross over to the surgical arm. Whether a degenerative meniscal tear or mechanical symptoms are present does not seem to make a difference to the long-term outcomes.
It is our role at the front line to make this clear to patients, because getting an arthroscopy is very easy but also has significant risks.
Re-alignment procedures such as high tibial osteotomy are brutal, but effective. They involve breaking the medial side of the tibia, holding it open with a plate, and allowing bone to fill the defect so that the leg is less bow legged. It gives an average of 12 years of pain relief before patients require total knee replacement. It is generally offered to active patients under the age of 55, with isolated medial compartment knee OA.
Uni-compartmental joint replacement can be done for either compartment. Again, the medial compartment is the best studied, and this provides long-term improved pain and function in these patients.Overall patients are very satisfied at an early stage following the procedure. Very active patients may be at risk of early prosthetic wear or loosening. The conversion to total knee replacement is made slightly harder due to loss of some bone around the components.
FUTURE DIRECTIONS
There are many innovative treatments under investigation that aim to target the pain generators, inflammatory and catabolic processes involved in knee OA.
Pain treatments with promise include monoclonal antibodies to nerve growth factors. Blockade using a monoclonal antibody lead to around 50% reduction in pain during walking. While this treatment appears promising for pain, it is still under investigation in clinical trials due to some concerns that it is associated with accelerated progression of knee OA.
Strontium has been plugged as a potential DMARD in OA, particularly for the “bone phenotype”, but there is no trustworthy evidence of this yet. Strontium reduces subchondral bone resorption, and is also thought to reduce matrix breakdown. There are industry-funded studies showing beneficial effects on radiographic OA progression, especially when bone marrow lesions were present. Effects on symptoms and function were minimal.
Fibroblast growth factor-18, or sprifermin, is an agent that is thought to reduce joint catabolism and improve matrix regeneration. When injected into joints, it has shown dose-dependent reductions in joint space narrowing, but only in the lateral compartment. It appears safe but there is no evidence of symptom or functional improvement.
Tissue engineering is making progress. The future of restoring articular cartilage likely comes in scaffolds lined with stem cells. Animal models have shown this process to be quite promising.
APPLYING THE EVIDENCE
If a patient is still struggling despite attempts at weight loss and progressive strengthening, what would you offer them?
The patient may well be asking about treatments that are generally not recommended. This kind of mismatch between public perception and reality is common. This will require some time to correct misconceptions and set realistic expectations. Being mindful of the patient’s goals, such as remaining active and continuing in a physical job, a range of treatment options become available.
The GP is instrumental in assessing the patient’s metabolic health and risk factors, including blood pressure, fasting glucose and insulin, waist circumference, renal function and lipid profile.
Hypertension and impaired renal function may be relative contra-indications to NSAIDs. In older patients, comorbidities may increase surgical risk. These factors put urgency on weight loss and exercise as key treatments, and make local treatments more appropriate, including topical and injectable treatments for pain.
The first question is whether the patient has really embraced the active treatments for knee OA. In the sports-medicine setting, there is a tendency to use innovative methods that engage the patient. Patients have difficulty appreciating the important effect of weight loss on their knee pain.
It can become much more tangible with a simple demonstration using buoyancy to take their body weight off. I like to do this using an “Alter-G” machine, which is a treadmill that is easily able to reduce the patient’s body weight by 10% while they walk. For patients with medial knee OA and varus knees, try them out with an offloader brace.
For patients with lateral knee OA and valgus knees, put an orthotic in their shoe. Seeing and feeling is believing, so this approach is much more motivating than asking patients to take your word for it.
Patients come to sport and exercise physicians for injections. Before offering anything invasive, a checklist of the evidence-based treatments should be completed.
Review of the patient’s KOOS score change from baseline helps quantify the effects of treatments on pain and function.
If a patient goes ahead with an injectable treatment, it needs to be combined with specific post-injection instructions.
In my practice, patients must take five days off any formal exercise. They must meet their rehabilitator one week following the injection to re-commence progressive strengthening. A review in four weeks generally allows an accurate assessment of the effect on any injection.
If there are any concerning symptoms (mainly locking), a lack of significant improvement or severe deterioration in symptoms and function, as measured by the KOOS score after six to eight weeks of conservative treatment, a surgical opinion is worthwhile.
These patients are very easy to refer to orthopaedic surgeons. But the evidence-based surgeon knows that, in most instances, surgery does not genuinely help the patient.
Dr David Samra is a senior specialist registrar in sports and exercise medicine, currently working with the Sydney Swans. He is also a trained physiotherapist.
References:
23. Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008 Dec;67(12):1716–23.
24. Roberts E, Delgado Nunes V, Buckner S, Latchem S, Constanti M, Miller P, et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis. 2016 Mar;75(3):552–9.
25. Derry S, Conaghan P, Da Silva JAP, Wiffen PJ, Moore RA. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016 Apr 22;4:CD007400.
26. Deal CL, Schnitzer TJ, Lipstein E, Seibold JR, Stevens RM, Levy MD, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991 Jun;13(3):383–95.
27. da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet Lond Engl. 2017 Jul 8;390(10090):e21–33.
28. Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015 Feb 17;162(4):276–86.
29. Aran S, Malekzadeh S, Seifirad S. A double-blind randomized controlled trial appraising the symptom-modifying effects of colchicine on osteoarthritis of the knee. Clin Exp Rheumatol. 2011 Jun;29(3):513–8.
30. Srivastava R, Das SK, Goel G, Asthana A, Agarwal GG. Does long term colchicine prevent degradation of collagen fiber network in osteoarthritis? Int J Rheum Dis. 2017 Mar 6;
31. Wang ZY, Shi SY, Li SJ, Chen F, Chen H, Lin HZ, et al. Efficacy and Safety of Duloxetine on Osteoarthritis Knee Pain: A Meta-Analysis of Randomized Controlled Trials. Pain Med Malden Mass. 2015 Jul;16(7):1373–85.
32. Atamaz FC, Durmaz B, Baydar M, Demircioglu OY, Iyiyapici A, Kuran B, et al. Comparison of the efficacy of transcutaneous electrical nerve stimulation, interferential currents, and shortwave diathermy in knee osteoarthritis: a double-blind, randomized, controlled, multicenter study. Arch Phys Med Rehabil. 2012 May;93(5):748–56.
33. Foster NE, Thomas E, Barlas P, Hill JC, Young J, Mason E, et al. Acupuncture as an adjunct to exercise based physiotherapy for osteoarthritis of the knee: randomised controlled trial. BMJ. 2007 Sep 1;335(7617):436.
34. Hunter D, Gross KD, McCree P, Li L, Hirko K, Harvey WF. Realignment treatment for medial tibiofemoral osteoarthritis: randomised trial. Ann Rheum Dis. 2012 Oct;71(10):1658–65.
35. Rodrigues PT, Ferreira AF, Pereira RMR, Bonfá E, Borba EF, Fuller R. Effectiveness of medial-wedge insole treatment for valgus knee osteoarthritis. Arthritis Rheum. 2008 May 15;59(5):603–8.
36. Jones A, Silva PG, Silva AC, Colucci M, Tuffanin A, Jardim JR, et al. Impact of cane use on pain, function, general health and energy expenditure during gait in patients with knee osteoarthritis: a randomised controlled trial. Ann Rheum Dis. 2012 Feb;71(2):172–9.
37. Lee YH, Woo J-H, Choi SJ, Ji JD, Song GG. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int. 2010 Jan;30(3):357–63.
38. Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G, CS/GS Combined Therapy Study Group. Combined Treatment With Chondroitin Sulfate and Glucosamine Sulfate Shows No Superiority Over Placebo for Reduction of Joint Pain and Functional Impairment in Patients With Knee Osteoarthritis: A Six-Month Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Arthritis Rheumatol Hoboken NJ. 2017 Jan;69(1):77–85.
39. Hill CL, March LM, Aitken D, Lester SE, Battersby R, Hynes K, et al. Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Ann Rheum Dis. 2015 Sep 9;annrheumdis – 2014–207169.
40. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005328.
41. Hepper CT, Halvorson JJ, Duncan ST, Gregory AJM, Dunn WR, Spindler KP. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg. 2009 Oct;17(10):638–46.
42. Hirsch G, Kitas G, Klocke R. Intra-articular corticosteroid injection in osteoarthritis of the knee and hip: factors predicting pain relief–a systematic review. Semin Arthritis Rheum. 2013 Apr;42(5):451–73.
43. McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, et al. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA. 2017 May 16;317(19):1967–75.
44. Saccomanno MF, Donati F, Careri S, Bartoli M, Severini G, Milano G. Efficacy of intra-articular hyaluronic acid injections and exercise-based rehabilitation programme, administered as isolated or integrated therapeutic regimens for the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc Off J ESSKA. 2016 May;24(5):1686–94.
45. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015 Jan 6;162(1):46–54.
46. Bannuru RR, Vaysbrot EE, Sullivan MC, McAlindon TE. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014 Apr;43(5):593–9.
47. Leighton R, Akermark C, Therrien R, Richardson JB, Andersson M, Todman MG, et al. NASHA hyaluronic acid vs. methylprednisolone for knee osteoarthritis: a prospective, multi-centre, randomized, non-inferiority trial. Osteoarthr Cartil OARS Osteoarthr Res Soc. 2014 Jan;22(1):17–25.
48. Printz JO, Lee JJ, Knesek M, Urquhart AG. Conflict of Interest in the Assessment of Hyaluronic Acid Injections for Osteoarthritis of the Knee: An Updated Systematic Review. J Arthroplasty. 2013 Sep;28(8, Supplement):30–3.e1.
49. Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis – meta-analysis. Osteoarthritis Cartilage. 2011 Jun;19(6):611–9.
50. DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2012 Jul;28(7):998–1009.
51. Laudy ABM, Bakker EWP, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015 May;49(10):657–72.
52. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013 Feb;41(2):356–64.
53. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of Intra-articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthrosc J Arthrosc Relat Surg. 2016 Mar;32(3):495–505.
54. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of Leukocyte Concentration on the Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis. Am J Sports Med. 2016 Mar 1;44(3):792–800.
55. Zhu Y, Yuan M, Meng HY, Wang AY, Guo QY, Wang Y, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013 Nov 1;21(11):1627–37.
56. Astolfi M, McGuire K, Kaminski TW. The effectiveness of autologous conditioned serum in the treatment of knee osteoarthritis. J Sport Rehabil. 2014 Nov;23(4):365–9.
57. Baltzer AWA, Moser C, Jansen SA, Krauspe R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthr Cartil OARS Osteoarthr Res Soc. 2009 Feb;17(2):152–60.
58. Imam MA, Holton J, Ernstbrunner L, Pepke W, Grubhofer F, Narvani A, et al. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. Int Orthop. 2017 Aug 13;
59. Amato B, Compagna R, Amato M, Butrico L, Fugetto F, Chibireva MD, et al. The role of adult tissue-derived stem cells in chronic leg ulcers: a systematic review focused on tissue regeneration medicine. Int Wound J. 2016 Dec;13(6):1289–98.
60. Wong KL, Lee KBL, Tai BC, Law P, Lee EH, Hui JHP. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2013 Dec;29(12):2020–8.
61. Koh Y-G, Kwon O-R, Kim Y-S, Choi Y-J. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2014 Nov;30(11):1453–60.
62. Xia P, Wang X, Lin Q, Li X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2015 May 6;39(12):2363–72.
63. Pas HI, Winters M, Haisma HJ, Koenis MJ, Tol JL, Moen MH. Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med. 2017 Aug;51(15):1125–33.
64. Osborne H, Anderson L, Burt P, Young M, Gerrard D. Australasian College of Sports Physicians-Position Statement: The Place of Mesenchymal Stem/Stromal Cell Therapies in Sport and Exercise Medicine. Clin J Sport Med Off J Can Acad Sport Med. 2016 Mar;26(2):87–95.
65. Osborne H, Castricum A. Change to Australasian College of Sport and Exercise Physicians-position statement: the place of mesenchymal stem/stromal cell therapies in sport and exercise medicine. Br J Sports Med. 2016 Oct;50(20):1229.
66. Yim J-H, Seon J-K, Song E-K, Choi J-I, Kim M-C, Lee K-B, et al. A comparative study of meniscectomy and nonoperative treatment for degenerative horizontal tears of the medial meniscus. Am J Sports Med. 2013 Jul;41(7):1565–70.
67. Herrlin SV, Wange PO, Lapidus G, Hållander M, Werner S, Weidenhielm L. Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five year follow-up. Knee Surg Sports Traumatol Arthrosc Off J ESSKA. 2013 Feb;21(2):358–64.
68. Katz JN, Brophy RH, Chaisson CE, de Chaves L, Cole BJ, Dahm DL, et al. Surgery versus Physical Therapy for a Meniscal Tear and Osteoarthritis. N Engl J Med. 2013 May 2;368(18):1675–84.
69. Kirkley A, Birmingham TB, Litchfield RB, Giffin JR, Willits KR, Wong CJ, et al. A Randomized Trial of Arthroscopic Surgery for Osteoarthritis of the Knee. N Engl J Med. 2008 Sep 11;359(11):1097–107.
70. Moseley JB, O’Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, et al. A Controlled Trial of Arthroscopic Surgery for Osteoarthritis of the Knee. N Engl J Med. 2002 Jul 11;347(2):81–8.
71. Reichenbach S, Rutjes AW, Nüesch E, Trelle S, Jüni P. Joint lavage for osteoarthritis of the knee. Cochrane Database Syst Rev. 2010;(5):CD007320.
72. Fu D, Li G, Chen K, Zhao Y, Hua Y, Cai Z. Comparison of high tibial osteotomy and unicompartmental knee arthroplasty in the treatment of unicompartmental osteoarthritis: a meta-analysis. J Arthroplasty. 2013 May;28(5):759–65.
73. Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the Treatment of Pain from Osteoarthritis of the Knee. N Engl J Med. 2010 Oct 14;363(16):1521–31.
74. Reginster J-Y, Badurski J, Bellamy N, Bensen W, Chapurlat R, Chevalier X, et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised placebo-controlled trial. Ann Rheum Dis. 2013 Feb;72(2):179–86.
75. Pelletier J-P, Roubille C, Raynauld J-P, Abram F, Dorais M, Delorme P, et al. Disease-modifying effect of strontium ranelate in a subset of patients from the Phase III knee osteoarthritis study SEKOIA using quantitative MRI: reduction in bone marrow lesions protects against cartilage loss. Ann Rheum Dis. 2015 Feb;74(2):422–9.
76. Lohmander LS, Hellot S, Dreher D, Krantz EFW, Kruger DS, Guermazi A, et al. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol Hoboken NJ. 2014 Jul;66(7):1820–31.
77. Diao H, Wang J, Shen C, Xia S, Guo T, Dong L, et al. Improved cartilage regeneration utilizing mesenchymal stem cells in TGF-beta1 gene-activated scaffolds. Tissue Eng Part A. 2009 Sep;15(9):2687–98.